
Efficacy and safety of anlotinib plus EGFR tyrosine kinase inhibitors in slow- or locally progressing non-small cell lung cancer after adjuvant therapy
Highlight box
Key findings
• This study evaluated the combination of anlotinib and endothelial growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small cell lung cancer (NSCLC) who developed resistance to postoperative EGFR-TKI adjuvant therapy. The median progression-free survival (PFS) was 9.5 months, with the 6-month and 12-month PFS rates being 70.8% and 47.9%, respectively. The median overall survival (OS) was 31 months, with high 6-month and 12-month OS rates of 91.7% and 85.4%, respectively. Treatment-related adverse events (TRAEs) were common, with hypertension being the most frequent.
What is known and what is new?
• Previous studies have explored the use of EGFR-TKIs in patients with NSCLC, but resistance often develops. Combination therapies with other agents, such as anlotinib, have shown promise in overcoming this resistance.
• This study adds evidence regarding the efficacy and safety of anlotinib combined with EGFR-TKIs in patients who have developed resistance after EGFR-TKI adjuvant therapy.
What is the implication, and what should change now?
• The findings suggest that anlotinib combined with EGFR-TKIs can extend PFS and OS in patients with NSCLC with EGFR-TKI resistance. This combination offers a potential therapeutic option for patients who otherwise have limited treatment options. Clinicians should consider this approach for patients exhibiting resistance to adjuvant therapy while also monitoring for manageable TRAEs. Further studies are needed to validate these findings and optimize treatment regimens.
Introduction
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have shown significant efficacy in patients with non-small cell lung cancer (NSCLC) harboring EGFR mutations (1). However, the efficacy of EGFR-TKIs varies among patients with NSCLC, with a minority achieving complete remission and others achieving partial remission (2). The iterative upgrading of EGFR-TKIs from the first to the third generation in clinical practice addresses the issue of diminished or lost efficacy (3). Although third-generation EGFR-TKIs outperform first- and second-generation drugs in terms of efficacy and toxicity, disease progression remains inevitable due to acquired resistance, imposing a substantial economic burden on society and patients (4). Previous studies have indicated that among the resistance mechanisms of first- and second-generation EGFR-TKIs, the T790M mutation represents the most common resistance alteration, whereas the resistance mechanisms of third-generation EGFR-TKIs are more complex, primarily involving EGFR C797S mutation, bypass activation pathways such as MET amplification, activation of downstream signaling pathways, or histological transformation. After resistance develops, clinical practice often involves increasing the daily dose of the targeted drug (5), combining it with antiangiogenic agents (6), or integrating other treatment modalities as proposed in studies such as FLAURA-2 (7) and MARIPOSA (8) to improve patient survival. Due to the complex and diverse mechanisms underlying EGFR-TKI resistance, clinical exploration of more effective therapeutic strategies remains ongoing (9,10).
Angiogenesis plays a crucial role in tumor growth and metastasis, and blocking this pathway has become a successful strategy in clinical cancer treatment. Combination therapy of EGFR-TKIs with anlotinib has emerged as a potential strategy for overcoming dr ug resistance and improving treatment outcomes in patients with EGFR-positive NSCLC (11). Anlotinib is a novel oral receptor TKI targeting vascular endothelial growth factor receptor (VEGFR) 2 and 3, fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptor (PDGFR) α and β, c-Kit, and Ret (12), exhibiting potent antitumor angiogenesis effects and inhibiting malignant tumor progression (13). Studies have demonstrated that the efficacy of anlotinib was favorable in the treatment of NSCLC and anlotinib therapy prolongs progression-free survival (PFS) and overall survival (OS) (13-15). Studies indicate that combining EGFR-TKIs with anlotinib inhibits tumors via multiple signaling pathways, enhancing efficacy in patients with EGFR-positive NSCLC (6,16-18). This combination therapy has demonstrated efficacy in inhibiting tumor cell proliferation, angiogenesis, and metastasis in EGFR-driven NSCLC models, indicating its potential clinical utility in overcoming resistance to EGFR-TKIs (11).
Given the promising preclinical data, evaluating the clinical efficacy and safety of combining anlotinib with EGFR-TKIs in patients with NSCLC who have developed resistance to EGFR-TKI adjuvant therapy is particularly important. However, few studies have assessed the efficacy and safety of combining anlotinib with EGFR-TKIs in patients with NSCLC resistant to EGFR-TKI adjuvant therapy. This study aimed to evaluate the efficacy and safety of combining anlotinib with EGFR-TKIs in patients with NSCLC who have developed resistance following EGFR-TKI adjuvant therapy after surgery. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-177/rc).
Methods
Patients
This retrospective, real-world study was conducted at the First Affiliated Hospital of Zhejiang University School of Medicine and included patients who underwent surgery received EGFR-TKI adjuvant therapy and subsequently developed resistance between January 2020 and December 2023. The criteria for diagnosing resistance included elevated blood carcinoembryonic antigen (CEA) levels (19), nodule changes [emergence of new high-risk nodules (for patients with only one primary nodule) or enlargement of existing high-risk nodules (for patients with multiple high-risk ground glass nodules)] according to the response evaluation criteria in solid tumor version 1.1 (RECIST 1.1), and metastasis (lymph nodes, bone, brain, or pleura). Elevated CEA was defined as blood CEA <10.0 ng/mL followed by consecutive measurements ≥10.0 ng/mL (with intervals of at least 1 month) or blood CEA ≥10.0 ng/mL with gradual increases in consecutive measurements (with intervals of at least 1 month). All patients underwent positron emission tomography (PET)–computed tomography (CT) after diagnosing resistance.
The inclusion criteria for patients were as follows: (I) age ≥18 and ≤75 years; (II) postoperative NSCLC; (III) genetic testing reporting EGFR exon 19 deletion or 21 L858R mutation; (IV) resistance to EGFR-TKI adjuvant therapy; (V) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and (VI) adequate organ function, including sufficient lung and heart function.
Patients with the following conditions were excluded: (I) lack of essential imaging evaluations at the study hospital; (II) presence of autoimmune diseases, infectious diseases, or other concomitant malignant tumors; (III) prior radiotherapy, chemotherapy, or immunotherapy; and (IV) previous or ongoing systemic immunosuppressive treatment.
Patient follow-up data were retrieved from the hospital’s medical record system and through telephone contact, with the latest follow-up cutoff date being March 25, 2024.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived for the retrospective data. This study received approval from the Ethics Review Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (No. IIT20240441A).
Treatment methods
The included patients were administered anlotinib (orally, days 1–14, once every 3 weeks, followed by a 7-day break) in addition to their original EGFR-TKI regimen. The initial dose of anlotinib (10 or 12 mg) was jointly determined by clinicians and pharmaceutical experts based on the patient’s condition. The dosage was reduced when patients experienced intolerable treatment-related adverse events (TRAEs). Patients were treated with anlotinib until progressive disease or TRAE intolerance occurred.
Efficacy and safety
The primary endpoint of this study was PFS, while secondary endpoints included 6- and 12-month PFS rates, OS, and safety. PFS was defined as the time from the initiation of anlotinib plus EGFR-TKI to disease progression [elevated blood CEA levels, nodule changes according to the RECIST 1.1, metastasis (lymph nodes, bone, brain, or pleura)] or death. OS was defined as the time from the start of anlotinib plus EGFR-TKI to death from any cause. TRAEs were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and R software version 4.1.2 (The R Foundation for Statistical Computing), while GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA) was used for plotting. Categorical variables are expressed as frequencies and percentages, while continuous variables are expressed as the median and interquartile range (IQR). The Fisher exact test or Pearson chi-square test was used to compare the baseline characteristics between the groups. The Kaplan-Meier method was used to evaluate the PFS and OS, and stratified log-rank tests were applied to compare the differences between the groups. The reverse Kaplan-Meier method was used to estimate the median follow-up time. The correlation between each study variable and survival was determined using stratified Cox proportional hazard models. The statistical significance threshold was set at P<0.05.
Results
Baseline characteristics of participants
From January 2020 to December 2023, 48 patients who underwent surgery and developed resistance to adjuvant therapy with EGFR-TKIs were included in the study. The baseline characteristics of the patients are shown in Table 1. The median age of the 48 patients was 68 years, with an equal distribution of males and females, each accounting for 50%. Genetic testing reports of patient’s tumor tissue indicated that 52.1% (25/48) of the patients had the L858R mutation, while 47.9% (23/48) had exon 19 deletions. And 4 patients had concurrent TP53 mutations, 1 patient had concurrent MET mutations, 1 patient had concurrent ALK, 1 patient had concurrent HER2. After surgery, 23 (47.9%) patients received first- or second-generation EGFR-TKI treatment, and 25 (52.1%) patients received third-generation EGFR-TKI therapy. The median interval time from surgery to resistance of adjuvant therapy was 20.0 months [95% confidence interval (CI): 14.0–33.5; range, 0.0–48.0]. After EGFR-TKI adjuvant therapy, 18 (37.5%) patients exhibited elevated blood CEA levels. Six patients (12.5%) developed new high-risk nodules, and seven (14.6%) had enlargement of existing ones. Seventeen (35.4%) patients had metastases (lymphadenopathy in seven patients, bone in three, brain in three, and pleura in four). All patients underwent next-generation sequencing (NGS) in liquid biopsy after diagnosing resistance. Among patients received first- or second-generation EGFR-TKI, 6 patients (6/23, 26.1%) were found to have an EGFR T790M mutation, and the others had an unknown resistance mechanism. For patients received third-generation EGFR-TKI therapy, 3 patients (3/25, 12.0%) were found to have an EGFR C797S mutation, and the others had an unknown resistance mechanism.
Table 1
| Characteristic | Value (N=48) |
|---|---|
| Age (years), median (IQR) | 68.0 (55.0–72.0) |
| Sex, n (%) | |
| Male | 24 (50.0) |
| Female | 24 (50.0) |
| ECOG performance status, n (%) | |
| 0 | 28 (58.3) |
| 1 | 20 (41.7) |
| Smoking status, n (%) | |
| Never | 40 (83.3) |
| Ever | 8 (16.7) |
| Drinking status, n (%) | |
| Never | 44 (91.7) |
| Ever | 4 (8.3) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 5 (10.4) |
| Hypertension | 15 (31.3) |
| Surgical method, n (%) | |
| Wedge resection | 15 (31.3) |
| Lobectomy | 33 (68.7) |
| Pathological stage, n (%) | |
| IIA | 16 (33.3) |
| IIB | 4 (8.3) |
| IIIA | 24 (50.0) |
| IIIB | 4 (8.3) |
| Pathological grade, n (%) | |
| G1 | 3 (6.3) |
| G2 | 17 (35.4) |
| G3 | 28 (58.3) |
| EGFR mutation subtype, n (%) | |
| Exon 19 deletion | 23 (47.9) |
| L858R mutation | 25 (52.1) |
| EGFR-TKI type, n (%) | |
| First-/second-generation TKI | |
| Icotinib | 16 (33.3) |
| Gefitinib | 5 (10.4) |
| Afatinib | 2 (4.2) |
| Third-generation TKI | |
| Furmonertinib | 3 (6.3) |
| Almonertinib | 1 (2.1) |
| Osimertinib | 21 (43.8) |
| Resistance manifestation, n (%) | |
| Elevated blood CEA level | 18 (37.5) |
| Nodule change | |
| Emergence of new nodule | 6 (12.5) |
| Enlargement of original nodule | 7 (14.6) |
| Metastasis | |
| Lymph node | 7 (14.6) |
| Bone | 3 (6.3) |
| Brain | 3 (6.3) |
| Pleura | 4 (8.3) |
CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IQR, interquartile range; TKI, tyrosine kinase inhibitor.
Adverse events
No previously undocumented TRAEs were observed in this study (Table 2). Overall, the incidence rates of any grade and grade ≥3 TRAEs were 75.0% (36/48) and 10.4% (5/48), respectively. The most common TRAEs were hypertension (17/48, 35.4%), proteinuria (15/48, 31.3%), rash (11/48, 22.9%), fatigue (5/48, 10.4%), and diarrhea (4/48, 8.3%). No new safety events were reported. Grade 1–2 TRAEs were the most common, with fewer patients experiencing grade 3 or 4 TRAEs (two cases of urine protein, two cases of hypertension, and one case of skin rash). All symptoms resolved after treatment. Dose reduction and discontinuation of anlotinib were reported in four (8.3%) and five (10.4%) patients, respectively. A summary of TRAEs is presented in Table 2.
Table 2
| Event | Any grade | Grade 3 or 4 |
|---|---|---|
| Any adverse events | 36 (75.0) | 5 (10.4) |
| Thrombocytopenia | 2 (4.2) | 0 (0.0) |
| Urine protein | 15 (31.3) | 2 (4.2) |
| Hepatic injury | 1 (2.1) | 0 (0.0) |
| Hypertension | 17 (35.4) | 2 (4.2) |
| Diarrhea | 4 (8.3) | 0 (0.0) |
| Nausea | 1 (2.1) | 0 (0.0) |
| Fatigue | 5 (10.4) | 0 (0.0) |
| Skin reaction | 11 (22.9) | 1 (2.1) |
| Oral ulcers | 2 (4.2) | 0 (0.0) |
Data are presented as n (%).
Survival
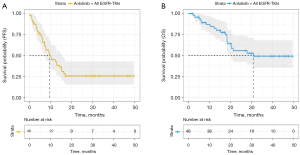
At the time of the data cutoff (March 2024), we successfully collected follow-up information for all patients. The median follow-up time was 33.3 months (95% CI: 23.2–43.3). Thirty-three patients experienced disease progression, and twenty patients died. Thirty-three patients went on to receive next-line therapy after progression on anlotinib plus EGFR TKI. After that, all of them chose immunotherapy with or without chemotherapy. The median PFS of all patients was 9.5 months (95% CI: 4.8–14.3), with a 6-month PFS rate of 70.8% and a 12-month PFS rate of 47.9% (Figure 1A). The median OS of all patients was 31.0 months [95% CI: not reached (NR)–NR], with 6-month and 12-month rates of 91.7% and 85.4%, respectively (Figure 1B).
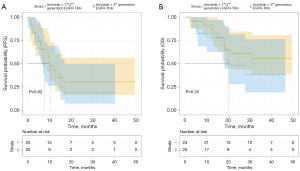
For patients previously treated with first- or second-generation EGFR-TKIs, the median PFS was 10.3 months (95% CI: 6.1–14.4), with a 6-month PFS rate of 69.6% and a 12-month PFS rate of 47.8% (Figure 2A). For patients previously treated with third-generation EGFR-TKIs, the median PFS was 7.7 months (95% CI: 4.8–10.6), with a 6-month PFS rate of 72.0% and a 12-month PFS rate of 48.0% (Figure 2A). For patients previously treated with first- or second-generation EGFR-TKIs, the median OS was NR, with a 6-month rate OS of 95.7% and a 12-month OS rate of 91.3% (Figure 2B). For patients previously treated with third-generation EGFR-TKIs, the median OS was 20.3 months (95% CI: 10.7–30.0), with a 6-month OS rate of 88.0% and a 12-month OS rate of 80.0% (Figure 2B).
For patients with elevated blood CEA levels, the median PFS was 9.1 months (95% CI: 2.7–15.5), with a 6-month PFS rate of 72.2% and a 12-month PFS rate of 50.0% (Figure 3A). For patients with nodule changes [emergence of new high-risk nodules (for patients with only one primary nodule) or enlargement of existing nodules (for patients with multiple high-risk ground glass nodules)], the median PFS was 10.3 months (95% CI: 3.1–17.4), with a 6-month PFS rate of 61.5% and a 12-month PFS rate of 46.2% (Figure 3A). For patients with metastases (lymphadenopathy, bone, brain, or pleura), the median PFS was 9.5 months (95% CI: 5.2–13.8), with a 6-month PFS rate of 76.5% and a 12-month PFS rate of 47.1% (Figure 3A). For patients with elevated blood CEA levels, the median OS was 27.9 months (95% CI: 14.7–41.1), with 6-month OS of 88.9% and a 12-month OS rate of 77.8% (Figure 3B). For patients with nodule changes [emergence of new high-risk nodules (for patients with only one primary nodule) or enlargement of existing nodules (for patients with multiple high-risk ground glass nodules)], the median OS was 20.3 months (95% CI: NR–NR), with a 6-month OS rate of 84.6% and a 12-month OS rate of 84.6% (Figure 3B). For patients with metastases (lymph node, bone, brain, or pleura), the median OS was NR (95% CI: NR–NR), with a 6-month OS rate of 100.0% and a 12-month OS rate of 88.2% (Figure 3B).

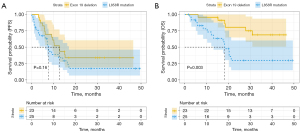
Univariate Cox regression analyses revealed no statistically significant correlations between these factors and PFS (Table 3). However, we found a statistically significant correlation between the EGFR mutation subtype and OS in the univariate Cox regression analyses (Table 4). We then included factors with P<0.1 in the multivariable Cox regression analyses. Compared to exon 19 deletion, the L858R mutation was associated with worse OS (HR 3.745, 95% CI: 1.411–9.943, P=0.008). The EGFR L858R mutation group had a median PFS of 7.6 months (95% CI: 6.7–8.6), while the median PFS of the EGFR exon 19 deletion mutation group was 12.7 months (95% CI: 8.6–16.8) (P=0.16) (Figure 4A). The EGFR L858R mutation group had a median OS of 18.3 months (95% CI: 15.6–21.0), while the median OS of the EGFR exon 19 deletion mutation group was not achieved (P=0.003) (Figure 4B).
Table 3
| Characteristic | Univariate analysis | |
|---|---|---|
| HR (95% CI) | P value | |
| Sex (vs. female) | 0.55 | |
| Male | 0.810 (0.408–1.606) | |
| Age (vs. ≤65 years) | 0.11 | |
| >65 years | 1.847 (0.877–3.888) | |
| ECOG PS (vs. 0) | 0.25 | |
| 1 | 1.496 (0.753–2.975) | |
| Smoking status (vs. never) | 0.18 | |
| Ever | 0.487 (0.171–1.389) | |
| Drinking status (vs. never) | 0.84 | |
| Ever | 0.884 (0.269–2.906) | |
| Hypertension (vs. no) | 0.85 | |
| Yes | 0.929 (0.441–1.957) | |
| Diabetes mellitus (vs. no) | 0.11 | |
| Yes | 2.192 (0.827–5.807) | |
| Surgical method (vs. wedge resection) | 0.82 | |
| Lobectomy | 0.916 (0.435–1.928) | |
| Pathological grade (vs. G1–G2) | 0.45 | |
| G3 | 1.309 (0.650–2.635) | |
| Pathological stage (vs. IIA–IIB) | 0.52 | |
| IIIA–IIIB | 0.794 (0.397–1.589) | |
| EGFR mutation subtype (vs. exon 19 deletion) | 0.16 | |
| L858R mutation | 1.635 (0.821–3.255) | |
| EGFR-TKI type (vs. third generation) | 0.62 | |
| First/second generation | 1.191 (0.559–2.369) | |
| Adverse events (vs. no) | 0.64 | |
| Yes | 1.236 (0.507–3.013) | |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HR, hazard ratio; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
Table 4
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (vs. female) | 0.66 | ||||
| Male | 0.822 (0.339–1.989) | ||||
| Age (vs. ≤65 years) | 0.40 | ||||
| >65 years | 1.507 (0.578–3.926) | ||||
| ECOG PS (vs. 0) | 0.71 | ||||
| 1 | 1.181 (0.490–2.848) | ||||
| Smoking status (vs. never) | 0.28 | ||||
| Ever | 0.447 (0.104–1.930) | ||||
| Drinking status (vs. never) | 0.98 | ||||
| Ever | 1.018 (0.236–4.394) | ||||
| Hypertension (vs. no) | 0.09 | 0.12 | |||
| Yes | 0.379 (0.125–1.144) | 0.413 (0.136–1.254) | |||
| Diabetes mellitus (vs. no) | 0.55 | ||||
| Yes | 1.458 (0.426–4.986) | ||||
| Surgical method (vs. wedge resection) | 0.69 | ||||
| Lobectomy | 0.827 (0.329–2.077) | ||||
| Pathological grade (vs. G1–G2) | 0.93 | ||||
| G3 | 0.960 (0.397–2.320) | ||||
| Pathological stage (vs. IIA–IIB) | 0.99 | ||||
| IIIA–IIIB | 1.002 (0.415–2.419) | ||||
| EGFR mutation subtype (vs. exon 19 deletion) | 0.006 | 0.008 | |||
| L858R mutation | 3.942 (1.487–10.456) | 3.745 (1.411–9.943) | |||
| EGFR-TKI type (vs. third generation) | 0.99 | ||||
| First/second generation | 1.002 (0.415–2.419) | ||||
| Resistance manifestation (vs. elevated blood CEA level) | 0.45 | ||||
| Nodule change | 0.870 (0.308–2.457) | ||||
| Metastasis | 0.512 (0.171–1.533) | ||||
| Adverse events (vs. no) | 0.89 | ||||
| Yes | 1.091 (0.315–3.779) | ||||
CEA, carcinoembryonic antigen; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HR, hazard ratio; OS, overall survival; TKI, tyrosine kinase inhibitor.
Discussion
The standard first-line treatment for patients with NSCLC harboring EGFR mutations is monotherapy with EGFR-TKIs. Although this provides significant survival benefits to patients, most will eventually experience disease progression over time (20,21). Due to the inevitable occurrence of resistance mutations during disease progression, clinical strategies, such as switching to a newer generation of EGFR-TKIs, are usually employed for subsequent treatment. Soria et al. (22), Park et al. (23), Urata et al. (24), and Wu et al. (25) have shown that in the first-line treatment of EGFR mutation-positive NSCLC, second- or third-generation EGFR-TKIs are more effective than are first-generation EGFR-TKIs, with significantly prolonged median PFS, similar safety profiles, and a lower incidence of serious adverse events. However, previous studies have indicated that when patients become resistant to standard TKI treatment, switching to second-generation EGFR-TKIs only provides a limited improvement in efficacy. Switching to third-generation TKIs, such as osimertinib, offers clinical benefits, but resistance may reoccur, requiring alternative treatment strategies such as combination chemotherapy (26-30). The mechanisms of acquired resistance to EGFR-TKIs are complex, and delaying resistance remains a major challenge in first-line treatment with EGFR-TKIs, for which there is no standard treatment paradigm. EGFR-TKI acquired resistance is composed of on-target (EGFR dependent), off-target (EGFR independent), and unknown (31). On-target acquired resistance of first- and second-generation EGFR TKI is relatively huge, and T790M mutation is the most common and the incidence rate is 50–60% (32). On-target acquired resistance of third-generation EGFR-TKIs was approximately 10–20% and C797S mutation was the most common (10). Off-target acquired resistance included many genes other than EGFR (33). Antiangiogenic therapy plays a crucial role in the treatment of EGFR-TKI resistance. For patients with disease progression after EGFR-TKI treatment, early use of combination therapy strategies may offer superior benefit as compared to EGFR-TKI monotherapy (15,34). Xiang et al. (35) compared the efficacy of EGFR-TKI monotherapy versus EGFR-TKIs combined with anlotinib, reporting that combination therapy achieved better PFS than did EGFR-TKI monotherapy. Our study found that when patients experienced disease progression due to resistance after EGFR-TKI treatment, combination therapy with anlotinib may prevent the need for an escalation of treatment strategies, prolong the treatment window, and provide more survival opportunities.
First- and second-generation EGFR-TKIs yield a median PFS between 9 and 13 months (36-41), while third-generation EGFR-TKIs yield a median PFS between 18 and 22 months (22,42). In our study, the median PFS was 9.5 months, with the median PFS of patients previously treated with first- and second-generation EGFR-TKIs being 10.3 months and that of those patients previously treated with third-generation EGFR-TKIs being 7.7 months. The median PFS yielded by the combination of anlotinib with EGFR-TKI for patients with NSCLC who developed resistance to EGFR-TKI adjuvant therapy following surgery and who were treated with the combination of anlotinib and EGFR-TKIs was similar to that of those treated with first- or second-generation EGFR-TKIs and about half of that of those treated with third-generation EGFR-TKIs. Therefore, for patients with NSCLC who develop resistance after undergoing EGFR-TKI adjuvant therapy after surgery, anlotinib plus EGFR-TKIs can prolong the treatment window. For patients previously treated with first- or second-generation EGFR-TKIs, we can delay the use of third-generation EGFR-TKIs and retain more treatment options. For patients previously treated with third-generation EGFR-TKIs, we can also delay the use of alternative treatment methods, such as chemotherapy and immunotherapy. In summary, whether using first- or second-generation EGFR-TKIs or third-generation EGFR-TKIs are applied, combining anlotinib with EGFR-TKIs can provide a greater opportunity for prolonged survival.
Our study defined resistance as an elevated blood CEA level, nodule changes [emergence of new high-risk nodules (for patients with only one primary nodule) or enlargement of existing nodules (for patients with multiple high-risk ground glass nodules)], and metastasis (lymph node, bone, brain, or pleura). We found the median PFS of patients with these three conditions of resistance was similar. The median PFS of patients with elevated blood CEA levels was 9.1 months, that for those with nodule changes [emergence of new high-risk nodules (for patients with only one primary nodule) or enlargement of existing nodules (for patients with multiple high-risk ground glass nodules)]was 10.3 months, and that for those with metastases (lymph node, bone, brain, or pleura) was 9.5 months. These results were similar to those observed for entire patient cohort. Therefore, anlotinib plus EGFR-TKIs is applicable to different manifestations of drug resistance.
This study included patients with EGFR exon 19 deletions and 21 L858R mutations. We found that the survival of patients with EGFR exon 19 deletions was better than that of patients with EGFR L858R mutations. The EGFR L858R mutation group had a median PFS of 7.6 months, whereas the median PFS of the EGFR exon 19 deletion mutation group was 12.7 months. The EGFR L858R mutation group had a median OS of 18.3 months, whereas the median OS of the EGFR exon 19 deletion group was not achieved. Therefore, anlotinib plus EGFR-TKIs may be more suitable for patients with EGFR exon 19 deletion mutations.
The toxicity observed in our study was consistent with that reported in other studies, and no new adverse events were identified (43,44). All symptoms resolved after treatment. The incidence rate of grade ≥3 TRAEs was 10.4% (two cases of urine protein, two cases of hypertension, and one case of skin rash). Dose reduction and discontinuation of anlotinib were reported in four (8.3%) and five (10.4%) patients, respectively. Our results indicated that anlotinib combined with the original EGFR-TKIs demonstrated controllable safety in patients who developed resistance after first-line EGFR-TKI treatment.
This retrospective study had some limitations, including a small sample size, a short postoperative follow-up period, and heterogeneity among patients and treatment modalities. Future research should adopt a prospective design and expand the sample size to validate these findings further, thereby enhancing the statistical power of the results and their external validity.
Conclusions
For patients with NSCLC who develop resistance to postoperative adjuvant therapy with EGFR-TKIs, the combination of anlotinib and EGFR-TKIs may provide good efficacy and manageable safety, offering these patients a longer treatment window and improved survival opportunities. However, large-scale randomized controlled trials are needed to confirm our findings.
Acknowledgments
We would like to thank the participants for their time and effort during the data collection phase. We also appreciate the support from the renowned Hu Jian Workstation in Taizhou, Zhejiang.
The abstract of this manuscript was presented as a poster at the World Conference on Lung Cancer (WCLC) in 2024 and published in the Journal of Thoracic Oncology (JTO) conference proceedings [https://www.jto.org/article/S1556-0864(24)01900-2/abstract].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-177/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-177/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-177/prf
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-177/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived for the retrospective data. This study received approval from the Ethics Review Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (No. IIT20240441A).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357-60. [Crossref] [PubMed]
- Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent 2024;4:47-53. [Crossref] [PubMed]
- Tfayli A, Mohty R. EGFR tyrosine kinase inhibitors in non-small cell lung cancer: treatment paradigm, current evidence, and challenges. Tumori 2021;107:376-84. [Crossref] [PubMed]
- Gao J, Li HR, Jin C, et al. Strategies to overcome acquired resistance to EGFR TKI in the treatment of non-small cell lung cancer. Clin Transl Oncol 2019;21:1287-301. [Crossref] [PubMed]
- Qi R, Fu X, Yu Y, et al. Efficacy and safety of re-challenging 160 mg furmonertinib for advanced NSCLC after resistance to third-generation EGFR-TKIs targeted agents: A real-world study. Lung Cancer 2023;184:107346. [Crossref] [PubMed]
- Lian Z, Du W, Zhang Y, et al. Anlotinib can overcome acquired resistance to EGFR-TKIs via FGFR1 signaling in non-small cell lung cancer without harboring EGFR T790M mutation. Thorac Cancer 2020;11:1934-43. [Crossref] [PubMed]
- Wang X, Huang Z, Lou R, et al. EP.12D.04 Treatment Pattern and Clinical Outcome in Chinese NSCLC Patients with MET Alterationwho Had Disease Progressionon EGFR-TKIs. Journal of Thoracic Oncology 2024;19:S651. [Crossref]
- Cho BC, Lu S, Felip E, et al. Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N Engl J Med 2024;391:1486-98. [Crossref] [PubMed]
- Chmielecki J, Gray JE, Cheng Y, et al. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat Commun 2023;14:1070. [Crossref] [PubMed]
- Passaro A, Jänne PA, Mok T, et al. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer 2021;2:377-91. [Crossref] [PubMed]
- Qin T, Liu Z, Wang J, et al. Anlotinib suppresses lymphangiogenesis and lymphatic metastasis in lung adenocarcinoma through a process potentially involving VEGFR-3 signaling. Cancer Biol Med 2020;17:753-67. [Crossref] [PubMed]
- Gao Y, Liu P, Shi R. Anlotinib as a molecular targeted therapy for tumors. Oncol Lett 2020;20:1001-14. [Crossref] [PubMed]
- Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018;118:654-61. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Efficacy and safety of third-line treatment with anlotinib in patients with refractory advanced non-small-cell lung cancer (ALTER-0303): a randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology 2017;18:S3. [Crossref]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Langer C, Soria JC. The role of anti-epidermal growth factor receptor and anti-vascular endothelial growth factor therapies in the treatment of non-small-cell lung cancer. Clin Lung Cancer 2010;11:82-90. [Crossref] [PubMed]
- Zhou HQ, Zhang YX, Chen G, et al. Gefitinib (an EGFR tyrosine kinase inhibitor) plus anlotinib (an multikinase inhibitor) for untreated, EGFR-mutated, advanced non-small cell lung cancer (FL-ALTER): a multicenter phase III trial. Signal Transduct Target Ther 2024;9:215. [Crossref] [PubMed]
- Lei T, Xu T, Zhang N, et al. Anlotinib combined with osimertinib reverses acquired osimertinib resistance in NSCLC by targeting the c-MET/MYC/AXL axis. Pharmacol Res 2023;188:106668. [Crossref] [PubMed]
- Chen HJ, Tu HY, Hu Y, et al. A phase II trial of anlotinib plus EGFR-TKIs in advanced non-small cell lung cancer with gradual, oligo, or potential progression after EGFR-TKIs treatment (CTONG-1803/ALTER-L001). J Hematol Oncol 2025;18:3. [Crossref] [PubMed]
- Cortot AB, Jänne PA. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev 2014;23:356-66. [Crossref] [PubMed]
- Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 2009;10:281-9. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Urata Y, Katakami N, Morita S, et al. Randomized Phase III Study Comparing Gefitinib With Erlotinib in Patients With Previously Treated Advanced Lung Adenocarcinoma: WJOG 5108L. J Clin Oncol 2016;34:3248-57. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. Erratum in: Lancet Oncol 2012;13:e186. [Crossref] [PubMed]
- Katakami N, Atagi S, Goto K, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 2013;31:3335-41. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Li YS, Jie GL, Wu YL. Novel systemic therapies in the management of tyrosine kinase inhibitor-pretreated patients with epidermal growth factor receptor-mutant non-small-cell lung cancer. Ther Adv Med Oncol 2023;15:17588359231193726. [Crossref] [PubMed]
- Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol 2016;9:34. [Crossref] [PubMed]
- Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol 2018;29:viii740. [Crossref]
- Yang Z, Tam KY. Combination Strategies Using EGFR-TKi in NSCLC Therapy: Learning from the Gap between Pre-Clinical Results and Clinical Outcomes. Int J Biol Sci 2018;14:204-16. [Crossref] [PubMed]
- Xiang H, Danna D, Xuefei C, et al. The efficacy and safety of adding anlotinib in gradual progression on third-generation EGFR-TKIs for EGFR-mutant advanced nonsmall cell lung cancer. Anticancer Drugs 2024;35:433-9. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [Crossref] [PubMed]
- Shao L, Wang W, Song Z, et al. The efficacy and safety of anlotinib treatment for advanced lung cancer. Onco Targets Ther 2019;12:6549-54. [Crossref] [PubMed]


