
Comparing neutrophil-to-lymphocyte ratio (NLR), absolute neutrophil count (ANC) and derived NLR as predictive biomarkers in first-line immunotherapy for non-small cell lung cancer: a retrospective study
Highlight box
Key findings
• Optimal cut-offs were identified, with neutrophil-to-lymphocyte ratio (NLR) showing consistent predictive value across subgroups.
• NLR, derived NLR (dNLR) and absolute neutrophil count (ANC) correlated with survival in patients receiving immune checkpoint inhibitors (ICIs) alone.
What is known and what is new?
• NLR, dNLR and ANC are reported as predictive of survival under immunotherapy. No study has compared their respective utilities in lung cancer.
• In this study, we compared predictive value of NLR, ANC and dNLR as predictive value of clinical outcomes for non-small cell lung cancer patients treated by ICI.
What is the implication, and what should change now?
• NLR performed consistently, while dNLR and ANC showed limited efficacy.
Introduction
Background
Lung cancer remains a significant global health burden, with its prevalence continuing to rise, accounting for 2.2 million new cases in 2020 (1). Among the various histological types, non-small cell lung cancer (NSCLC) predominates, comprising 80% of cases. Unfortunately, NSCLC is often diagnosed at advanced stages, contributing to its poor prognosis (2). In recent decade, the treatment landscape for advanced and metastatic NSCLC has witnessed a paradigm shift with the advent of immunotherapy, particularly immune checkpoint inhibitors (ICIs). These monoclonal antibodies, by targeting immune checkpoints such as programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1), unleash the host immune response against tumor cells, leading to potential durable clinical responses (3).
Despite the transformative impact of immunotherapy on NSCLC management, the selection of patients who will benefit the most remains a clinical challenge (4). Currently, PD-L1 expression assessed via immunohistochemistry serves as the most utilized biomarker for predicting immunotherapy response. However, its limitations, including incomplete prediction and inter-assay variability, underscore the need for alternative predictive biomarkers (5-7).
Rationale and knowledge gap
While several proposed biomarkers such as tumor mutational burden (TMB) and tumor infiltrating lymphocytes (TILs) necessitate invasive tissue management for assessment, there is a special interest for serum-based markers due to their cost-effectiveness and ease of use (5). Among these emerging biomarkers, the systemic inflammatory response, reflected by parameters such as the neutrophil-to-lymphocyte ratio (NLR), absolute neutrophil count (ANC) and derived NLR (dNLR), has gained significant interest (8). Elevated levels of NLR, ANC and dNLR have consistently been associated with poor clinical outcomes, impacting both progression-free survival (PFS) and overall survival (OS) (9-13). These markers have also been integrated into composite score, such as the systemic immune inflammatory index (SII) (14,15), lung immune prognostic index (LIPI) (16), Lung Immune Therapy Evaluation (LITE) (17) or lung immuno-oncology prognostic score (LIPS-3) (18), underscoring their significant potential. Despite their potential, the incorporation of NLR, ANC and dNLR into clinical practice has been hampered by inconsistent threshold values across studies. Indeed, the thresholds for NLR vary in the literature from 2.8 to 5. There is also a large variation in the proposed thresholds for dNLR, ranging from 2.2 to 3.8 or ANC ranging from 5 to 7.5 (19).
Moreover, while several studies have individually investigated the prognostic significance of NLR and dNLR, a direct comparison between these two biomarkers in the context of NSCLC immunotherapy remains lacking. Notably, the hypothesis suggesting dNLR as a more comprehensive reflection of tumor inflammation due to its inclusion of all white blood cell (WBC) subtypes, including monocytes (10), underscores the importance of such a comparative analysis. In addition to individual biomarker analyses, the majority of studies focused on populations at the metastatic stage receiving immunotherapy alone (10,20), with limited investigation into the context of combining immunotherapy with chemotherapy.
Objective
Therefore, the primary objective of this retrospective study was to bridge this gap by directly comparing NLR, ANC and dNLR as predictive biomarkers in the context of first-line immunotherapy for NSCLC. By utilizing the most commonly proposed cut-off values for each biomarker, we aimed to elucidate their respective prognostic implications and potential utility in guiding treatment decisions.
Through this investigation, we seek to contribute valuable insights into the predictive value of NLR, ANC and dNLR, aiding in the optimization of personalized treatment approaches for patients with advanced NSCLC undergoing immunotherapy. We present this article in accordance with the TRIPOD checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-808/rc).
Methods
Patients’ selection
A total of 70 patients diagnosed with metastatic NSCLC without actionable genetic alterations (AGAs) treated with first line immunotherapy (either as monotherapy or combined with chemotherapy) between September 2015 and March 2023 were included in this retrospective single-center study conducted at the University Hospital of Reims, France.
The inclusion criteria were as follows: (I) patients aged 18 years and above, with metastatic NSCLC treated at the University Hospital of Reims (France); (II) first line treatment by immunotherapy (monotherapy or combined with chemotherapy); (III) availability of clinical data and hematological values before pre-immunotherapy treatment. Exclusion criteria were: (I) histology inconsistent with NSCLC; (II) NSCLC with AGAs.
Information regarding baseline clinical characteristics, including age, gender, Eastern Cooperative Oncology Group-performance status (ECOG-PS), histology, TNM (tumor-node-metastasis) stage derived from pre-therapeutic positron emission tomography/computed tomography (PET/CT) scans, and smoking status, along with PD-L1 tumor proportion score (TPS), initiation date of the first treatment course, treatment line, specific ICI administered, initial follow-up date, and either the date of mortality or the most recent contact, were extracted from the patients’ medical records.
Survival analysis
For all included patients, comprehensive clinical and biological data were collected prior to the initiation of immunotherapy. In accordance with good clinical practice, immunotherapy was thereafter continued until progression, or toxicity, occurred. PFS was defined as the duration from the first cycle of treatment until either disease progression (as determined by CT scan) or death/last follow-up. OS was calculated as the period between the first treatment cycle and death or last follow-up. Assessment of treatment response relied on the RECIST (Response Evaluation Criteria in Solid Tumors) criteria, specifically RECIST 1.1 as outlined by Eisenhauer 2009 RECIST 1.1 (21). Complete response (CR) was defined as the disappearance of all target lesions, while partial response (PR) was characterized by a reduction of 30% or more in the total sum of the diameters of target lesions. Progressive disease (PD) was identified by a 20% or more increase in the total sum of the diameters of target lesions. Stable disease (SD) was documented for patients whose tumors did not exhibit sufficient shrinkage to meet the criteria for PR or sufficient growth to qualify for PD. In instances where CT evaluation was not feasible due to rapid disease progression or deterioration in the patient’s clinical condition, assessment of response was conducted through clinical and laboratory evaluations. These patients were thus analyzed for OS but were excluded from the PFS analysis per-RECIST.
NLR, ANC and dNLR assessments
To assess NLR and dNLR ratios, we used the patient’s biology performed a few days before the start of immunotherapy or chemo-immunotherapy, including hematological parameters [WBC count, absolute lymphocyte count (ALC), ANC]. NLR and dNLR were calculated as follows, respectively: NLR = ANC/ALC; dNLR = ANC/(WBC − ALC). Cuts off values used were 5 for NLR, 7.5 for ANC and 2.5 for dNLR based on literature analysis (19). Optimal cut-off values were assessed by receiver operating characteristic (ROC) analysis for both NLR, ANC and dNLR concerning PFS and OS.
Statistical analysis
The data were presented as median and range or counts and percentages for quantitative and qualitative variables, respectively. Associations between features were examined using the Fisher test. Quantitative data underwent analysis using non-parametric tests (Mann-Whitney or Kruskal-Wallis as appropriate) to assess significance across different conditions. Survival analysis was conducted using the Kaplan-Meier method and Cox regression as appropriate. According to the collinearity between NLR and dNLR (r=0.556, P<0.001), multivariate regression models were treated separately. The variables with a P value <0.10 in univariate analysis were integrated into multivariate models. In all exploratory analyses, results with a two-sided P value of ≤0.05 were considered significant. XLSTAT software (version 2022.4.1, Addinsoft Company, Paris, France) was employed for data analysis and formatting.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional committee board of University Hospital of Reims’ data protection departments (No. MR004160420221). In accordance with French law, an information notice was sent to all patients to fulfill the requirements for non-opposition.
Results
Patient’s characteristics
Among 230 patients treated with ICI between September 2015 and March 2023, 71 patients were eligible based on exclusion and inclusion criteria. Following the screening of these 71 patients (Figure 1), one patient was excluded due to chronic lymphocytic leukaemia, which impaired the interpretation of WBC counts. Thirty-two patients were treated with ICI alone, while 38 patients received chemo-immunotherapy as first-line treatment. Baseline patient characteristics are detailed in Table 1.

Table 1
| Variables | Values |
|---|---|
| Age (years) | 65.5 [40–88] |
| <65 | 34 (48.6) |
| ≥65 | 36 (51.4) |
| Male/female | 47/23 |
| Smoking history | |
| Never/former | 49 (70.0) |
| Current smokers | 21 (30.0) |
| PD-L1 expression (TPS) | 50 [0–100] |
| PD-L1 negative | 21 (30.0) |
| PD-L1 positive | 49 (70.0) |
| Histological sub-type | |
| Adenocarcinoma | 58 (82.9) |
| Squamous | 9 (12.9) |
| Other | 3 (4.2) |
| Stage† | |
| IIIA/IIIB | 3 (4.3) |
| IV | 67 (95.7) |
| KRAS status‡ (n=58) | |
| Wild type | 19 (32.8) |
| Mutated | 24 (41.4) |
| NA or unknown | 15 (25.9) |
| STK11 status‡ (n=58) | |
| Wild type | 45 (77.6) |
| Mutated | 9 (15.5) |
| NA or unknown | 4 (6.9) |
| Regimen | |
| ICI alone | 32 (45.7) |
| CT-ICI | 38 (54.3) |
| ECOG-PS | |
| 0–1 | 57 (81.4) |
| 2 | 13 (18.6) |
| Metastatic location | |
| Liver metastasis | 8 (11.4) |
| CNS metastasis | 10 (14.3) |
| Biological ratio | |
| ANC | 7.50 [1.10–52.90] |
| NLR | 4.49 [1.04–40.99] |
| dNLR | 2.29 [0.87–11.26] |
| Follow-up (days) | 336 [8–1,605] |
Data are presented as effective (percentage), median [range], or ratio, as appropriate. †, eighth edition of the TNM classification for lung cancer; ‡, among adenocarcinoma (n=58). ANC, absolute neutrophil count; CNS, central nervous system; CT-ICI, chemo-immunotherapy; dNLR, derived neutrophil-to-lymphocyte ratio; ECOG-PS, Eastern Cooperative Oncology Group-performance status; ICI, immune checkpoint inhibitor; NA, not applicable; NLR, neutrophil-to-lymphocyte ratio; PD-L1, programmed death-ligand 1; TNM, tumor-node-metastasis; TPS, tumor proportion score.
The median age was 65.5 (range, 40–88) years, comprising 47 men and 23 women. PD-L1 expression was high (i.e., TPS ≥50%) in 61.4% of cases. Most patients had adenocarcinoma (82.9%); non-squamous non-adenocarcinoma cases included two cases of undifferentiated lung carcinoma and one case of pleomorphic carcinoma. Central nervous system (CNS) metastasis and liver metastasis accounted for 14.3% and 11.4% of cases, respectively. Among adenocarcinoma, KRAS and STK11 mutations were observed in 24 patients (41.4%), and 15.5%, respectively.
Clinical associations with NLR, ANC or dNLR
We initially analyzed potential associations between NLR, ANC or dNLR and cofactors known to influence clinical outcomes, including age, PD-L1 expression, or ECOG-PS. Neither NLR, dNLR nor ANC showed any associations with the evaluated cofactors (Table 2).
Table 2
| Factors | NLR | dNLR | ANC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NLR <5 | NLR ≥5 | P value | dNLR <2.5 | dNLR ≥2.5 | P value | ANC <7.5 | ANC ≥7.5 | P value | |||
| Age | 0.98 | 0.66 | 0.63 | ||||||||
| ≥65 years | 20 [51] | 16 [52] | 22 [54] | 14 [48] | 19 [54] | 17 [49] | |||||
| <65 years | 19 [49] | 15 [48] | 19 [46] | 15 [52] | 16 [46] | 18 [51] | |||||
| Smoking history | 0.35 | 0.92 | 0.43 | ||||||||
| Never/former | 25 [64] | 24 [77] | 29 [71] | 20 [69] | 26 [74] | 23 [66] | |||||
| Current | 14 [36] | 7 [23] | 12 [29] | 9 [31] | 9 [26] | 12 [34] | |||||
| PD-L1 expression (TPS) | 0.14 | 0.93 | 0.10 | ||||||||
| PD-L1 negative | 15 [38] | 6 [19] | 13 [33] | 8 [27] | 13 [38] | 7 [20] | |||||
| PD-L1 positive | 24 [62] | 25 [81] | 27 [68] | 22 [73] | 21 [62] | 28 [80] | |||||
| Histological sub-type | 0.15 | 0.84 | 0.72 | ||||||||
| Non-squamous | 32 [82] | 29 [94] | 36 [88] | 25 [86] | 30 [86] | 31 [89] | |||||
| Squamous | 7 [18] | 2 [6] | 5 [12] | 4 [14] | 5 [14] | 4 [11] | |||||
| Regimen | 0.69 | 0.90 | 0.63 | ||||||||
| ICI alone | 17 [44] | 15 [48] | 19 [46] | 13 [45] | 15 [43] | 17 [49] | |||||
| CT-ICI | 22 [56] | 16 [52] | 22 [54] | 16 [55] | 20 [57] | 18 [51] | |||||
| ECOG-PS | 0.44 | 0.10 | 0.76 | ||||||||
| 0–1 | 33 [85] | 24 [77] | 36 [88] | 21 [72] | 29 [83] | 28 [80] | |||||
| 2 | 6 [15] | 7 [23] | 5 [12] | 8 [28] | 6 [17] | 7 [20] | |||||
| Metastatic location | |||||||||||
| Liver metastasis | 4 [10] | 4 [13] | 0.73 | 5 [12] | 3 [10] | 0.81 | 3 [9] | 5 [14] | 0.45 | ||
| CNS metastasis | 5 [13] | 5 [16] | 0.69 | 4 [10] | 6 [21] | 0.20 | 3 [9] | 7 [20] | 0.17 | ||
| Response at R1 | 0.23 | 0.02 | 0.39 | ||||||||
| CR/PR | 16 [41] | 9 [29] | 17 [41] | 8 [28] | 14 [40] | 11 [31] | |||||
| SD | 13 [33] | 8 [26] | 15 [37] | 6 [21] | 12 [34] | 9 [26] | |||||
| PD | 8 [21] | 8 [26] | 8 [20] | 8 [28] | 7 [20] | 9 [26] | |||||
| NA | 2 [5] | 6 [19] | 1 [2] | 7 [24] | 2 [6] | 6 [17] | |||||
| Best response | 0.16 | 0.06 | – | ||||||||
| CR/PR | 21 [54] | 11 [35] | 22 [54] | 10 [34] | |||||||
| SD | 6 [15] | 3 [10] | 7 [17] | 2 [7] | |||||||
| PD | 8 [21] | 8 [26] | 8 [20] | 8 [28] | |||||||
| NA | 4 [10] | 9 [29] | 4 [10] | 9 [31] | |||||||
Data are presented as n [%]. P value was considered significant if <0.05. CNS, central nervous system; CR/PR, complete response/partial response; CT-ICI, chemo-immunotherapy; dNLR, derived neutrophil-to-lymphocyte ratio; ECOG-PS, Eastern Cooperative Oncology Group-performance status; ICI, immune checkpoint inhibitor; NA, not applicable; NLR, neutrophil-to-lymphocyte ratio; PD, progressive disease; PD-L1, programmed death-ligand 1; R1, first re-evaluation; SD, stable disease; TPS, tumor proportion score.
However, dNLR was significantly associated with response at the first re-evaluation (P=0.02), with a similar trend observed for the best tumoral response (P=0.06).
Subsequently, we investigated the predictive capabilities of NLR, ANC and dNLR for patients diagnosed with NSCLC who underwent first-line immunotherapy. We evaluated the potential prognostic significance of each biomarker individually for both PFS and OS. Patients with NLR ≥5 experienced significantly lower PFS compared to those with NLR <5 (P=0.03) (Figure 2A) and poorer OS (P<0.001, Figure 2B). Conversely, a high dNLR (defined by a cutoff ≥2.5) did not demonstrate significance in identifying patients with poorer PFS among those receiving first-line immunotherapy (P=0.11) (Figure 2C) whereas a high dNLR was associated with poorer OS (P=0.008, Figure 2D). Similarly, an elevated ANC (≥7.5) also failed to identify patients with poor PFS (P=0.37) (Figure 2E) whereas a high ANC was associated with poorer OS (P=0.02, Figure 2F). These preliminary findings suggest that the NLR cut-off of 5 appears relevant as anticipated, whereas the expected significance for the dNLR cut-off of 2.5 or the ANC cut-off of 7.5 may be more limited.

Determination of optimal cut-off values for NLR, ANC and dNLR
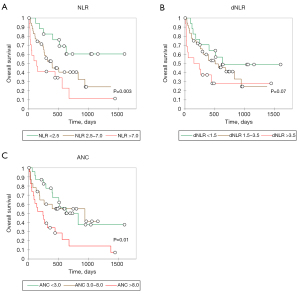
To address potential biases associated with the chosen cut-off values, we conducted comparisons using alternative cut-offs for NLR and dNLR. In our previous analyses, we utilized cut-off values commonly reported in the literature [5 for NLR, 2.5 for dNLR and 7.5 for ANC (19)]. Patients were categorized into three groups based on their baseline first and third quartiles for NLR (<2.5; 2.5–7; >7), dNLR (<1.5; 1.5–3.5; >3.5) or ANC (<3; 3–8; >8) levels, allowing for a comprehensive exploration of their survival trends (Figure 3). For NLR, we observed a distribution comprising three distinct curves with an effect inversely proportional to OS (NLR <2.5 vs. NLR 2.5–7.0: P=0.003). While this trend was not significantly evident for dNLR, there was a suggestive trend (P=0.07) indicating lower efficacy in predicting clinical outcomes with the dNLR variable. Similarly, for ANC, a global effect inversely proportional to OS was also observed (P=0.01), although the curves overlapped considerably for patient groups with ANC <3 and ANC between 3 and 8, indicating lower efficacy in predicting clinical outcomes with the ANC variable compared to NLR, where distinct and non-overlapping curves allowed for a clearer stratification of patient outcomes.
Using the ROC method, we determined optimal cut-off values. Interestingly, the optimal cut-off value for NLR to predict PFS was 5.0, as previously proposed [area under the curve (AUC) =0.570]. For dNLR, the optimal cut-off value to predict PFS was 1.786 but did not achieve statistical significance (AUC =0.552, P=0.41) while for ANC, the optimal cut-off value was 11.060, also without statistical significance (AUC =0.573, P=0.11) (Figure S1). Similar analyses were conducted for OS prediction. The optimal cut-off values for NLR, ANC and dNLR were 2.714 (AUC =0.683), 9.000 (AUC =0.683) and 2.496 (AUC =0.610), respectively, with no statistical difference observed (P<0.05 for all AUC comparisons) (Figure S2).
Clinical outcomes by NLR, ANC or dNLR
Subsequently, we conducted Cox regressions for NLR, ANC or dNLR individually, treating each biomarker as a continuous variable. It was observed that neither biomarker was significantly associated with any clinical, biological, or histological cofactor.
Cox regression models were applied to assess PFS and OS (Table 3), revealing a significant association in univariate analysis with ANC (P<0.001), NLR (P<0.001), dNLR (P=0.009), and liver metastasis (P=0.03) in OS.
Table 3
| Factors | Progression-free survival | Overall survival | |||
|---|---|---|---|---|---|
| Univariate HR (95% CI) | P value | Univariate HR (95% CI) | P value | ||
| Age | 0.982 (0.951–1.014) | 0.28 | 0.976 (0.947–1.006) | 0.12 | |
| Smoking habit (former vs. current smokers) | 1.019 (0.513–2.025) | 0.96 | 0.968 (0.481–1.947) | 0.93 | |
| ECOG-PS (0–1 vs. 2) | 1.310 (0.599–2.860) | 0.50 | 1.598 (0.780–3.272) | 0.20 | |
| Histological sub-type | 0.953 (0.398–2.284) | 0.92 | 0.680 (0.265–1.744) | 0.42 | |
| PD-L1 expression (TPS) | 0.998 (0.989–1.006) | 0.66 | 0.998 (0.989–1.006) | 0.66 | |
| Liver metastasis | 2.508 (0.861–7.301) | 0.09 | 2.571 (1.124–5.882) | 0.03 | |
| CNS metastasis | 1.264 (0.496–3.220) | 0.62 | 1.229 (0.516–2.928) | 0.64 | |
| Regimen (ICI vs. CT-ICI) | 0.901 (0.474–1.711) | 0.75 | 1.093 (0.577–2.071) | 0.78 | |
| ANC | 1.060 (1.018–1.103) | 0.005 | 1.069 (1.037–1.102) | <0.001 | |
| NLR | 1.084 (1.023–1.148) | 0.006 | 1.094 (1.053–1.137) | <0.001 | |
| dNLR | 1.210 (1.000–1.463) | 0.049 | 1.23 (1.053–1.437) | 0.009 | |
P value was considered significant if <0.05. ANC, absolute neutrophil count; CI, confidence interval; CNS, central nervous system; CT-ICI, chemo-immunotherapy; dNLR, derived neutrophil-to-lymphocyte ratio; ECOG-PS, Eastern Cooperative Oncology Group-performance status; HR, hazard ratio; ICI, immune checkpoint inhibitor; NLR, neutrophil-to-lymphocyte ratio; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
There was also a significant association in univariate analysis with NLR (P=0.006), ANC (P=0.005) and dNLR (P=0.049) in PFS. However, no significant association was observed with age, ECOG-PS, or CNS metastasis, in OS or PFS. Among responder patients, NLR, ANC and dNLR were not associated with response duration (P=0.62, P=0.93, and P=0.37, respectively) (data not shown).
As anticipated, we observed a significant collinearity between NLR and dNLR (r=0.556, P<0.001, data not shown) or NLR and ANC (r=0.806, P<0.001, data not shown) and ANC and dNLR (r=0.646, P<0.001, data not shown). Due to theses correlations, multivariate regression models had to be conducted separately for NLR, ANC and dNLR.
Subsequently, we performed multivariate analysis to assess the factors influencing PFS or OS, incorporating the presence of liver metastases in NLR, ANC or dNLR models, as appropriate (Table 4).
Table 4
| Factors | PFS | OS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NLR | dNLR | ANC | NLR | dNLR | ANC | ||||||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||||
| Liver metastasis | 2.043 (0.690–6.048) |
0.20 | 3.096 (1.077–8.902) |
0.04 | 1.971 (0.649–5.987) |
0.23 | 2.954 (1.401–6.230) |
0.004 | 3.232 (1.405–7.434) |
0.006 | 2.145 (0.927–4.961) |
0.07 | |||||
| ANC | – | – | 1.054 (1.011–1.099) |
0.01 | – | – | 1.066 (1.033–1.100) |
<0.001 | |||||||||
| NLR | 1.079 (1.016–1.145) |
0.01 | – | – | 1.097 (1.007–1.195) |
0.03 | – | – | |||||||||
| dNLR | – | 1.245 (1.026–1.512) |
0.03 | – | – | 1.275 (1.088–7.434) |
0.003 | – | |||||||||
P value was considered significant if <0.05. ANC, absolute neutrophil count; CI, confidence interval; dNLR, derived neutrophil-to-lymphocyte ratio; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PFS, progression-free survival.
Consequently, we observed independent significance for both NLR, ANC and dNLR in predicting PFS or OS among patients undergoing first-line immunotherapy.
dNLR, ANC and NLR comparisons for PFS
Subsequently, we investigated potential variations in significance and relevance for each biomarker across various subgroups of interest. Specifically, we explored the potential variation in prediction capacity for NLR, ANC and dNLR between patients treated with ICI alone or combined with chemo-immunotherapy. We hypothesized that NLR, ANC or dNLR might be intricately linked with ICI response and that chemotherapy combination could diminish their respective prediction abilities.
We observed a trend in NLR predicting PFS for patients undergoing ICI monotherapy (P=0.07), which was similar to the trend observed in patients receiving combined chemotherapy and ICI treatment (P=0.19) (Figure 4A,4B). Regarding dNLR, no significant association was found between dNLR levels and patients treated with either ICI monotherapy (P=0.21) or chemo-immunotherapy (P=0.31) (Figure 4C,4D). For ANC, no significant association was observed between ANC levels and patients receiving either ICI monotherapy (P=0.54) or chemo-immunotherapy (P=0.53) (Figure 4E,4F).

Table 5 presents comparisons between NLR, ANC and dNLR concerning PFS in other subgroups of interest.
Table 5
| Subgroups | NLR | dNLR | ANC | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age | ||||||||
| ≥65 years | 1.109 (1.034–1.189) | 0.004 | 1.387 (1.052–1.830) | 0.02 | 1.069 (1.016–1.125) | 0.009 | ||
| <65 years | 1.014 (0.891–1.153) | 0.84 | 1.067 (0.818–1.391) | 0.63 | 1.056 (0.961–1.160) | 0.25 | ||
| Smoking history | ||||||||
| Current smokers | 1.106 (0.889–1.377) | 0.36 | 1.726 (0.848–3.512) | 0.13 | 1.091 (0.936–1.272) | 0.26 | ||
| Former smokers | 1.098 (1.029–1.172) | 0.005 | 1.206 (0.969–1.502) | 0.10 | 1.066 (1.018–1.116) | 0.006 | ||
| PD-L1 expression | ||||||||
| Negative (TPS <1%) | 0.313 (0.941–1.833) | 0.11 | 1.487 (0.693–3.193) | 0.31 | 1.195 (0.885–1.613) | 0.24 | ||
| Positive (TPS ≥1%) | 1.072 (1.008–1.141) | 0.03 | 1.179 (0.936–1.484) | 0.16 | 1.058 (1.014–1.104) | 0.008 | ||
| Histological sub type | ||||||||
| Non-Sq NSCLC | 1.084 (1.017–1.155) | 0.01 | 1.173 (0.953–1.443) | 0.13 | 1.058 (1.014–1.105) | 0.009 | ||
| Sq-NSCLC | 1.173 (0.786–1.749) | 0.44 | 1.376 (0.709–2.673) | 0.35 | 1.248 (0.909–1.714) | 0.17 | ||
| Regimen | ||||||||
| ICI alone | 1.063 (0.998–1.132) | 0.056 | 1.170 (0.930–1.472) | 0.18 | 1.051 (1.005–1.099) | 0.03 | ||
| CT-ICI | 1.114 (0.977–1.271) | 0.11 | 1.413 (0.973–2.052) | 0.07 | 1.058 (0.976–1.147) | 0.17 | ||
| ECOG-PS | ||||||||
| 0–1 | 1.089 (1.020–1.164) | 0.01 | 1.247 (1.023–1.520) | 0.03 | 1.064 (1.016–1.114) | 0.007 | ||
| ≥2 | 1.081 (0.918–1.273) | 0.35 | 0.850 (0.433–1.668) | 0.64 | 1.044 (0.947–1.150) | 0.39 | ||
P value was considered significant if <0.05. ANC, absolute neutrophil count; CI, confidence interval; CT-ICI, chemo-immunotherapy; dNLR, derived neutrophil-to-lymphocyte ratio; HR, hazard ratio; ICI, immune checkpoint inhibitor; NLR, neutrophil-to-lymphocyte ratio; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1; ECOG-PS, Eastern Cooperative Oncology Group-performance status; Sq, squamous cell carcinoma; TPS, tumor proportion score.
The hazard ratio (HR) was consistently positive across all subgroups for NLR regarding PFS, with statistical significance observed for certain characteristics. A higher NLR was significantly associated with shorter PFS in subgroups of patients equal to or older than 65 years [HR =1.109; 95% confidence interval (CI): 1.034–1.189; P=0.004] or in patients with ECOG-PS 0–1 (HR =1.089; 95% CI: 1.020–1.164; P=0.01). These findings were consistent with higher dNLR, also linked to shorter PFS in these two subgroups (age ≥65 years and ECOG-PS 0–1, with respective P=0.02 and P=0.03). Similarly, elevated ANC was associated with shorter PFS in the same subgroups (P=0.009 and P=0.007).
Similar results were observed for dNLR, with an overall positive HR across all subgroups and statistical significance for certain characteristics.
In contrast to dNLR, associations between lower NLR and improved PFS were significantly observed in PD-L1-positive patients (HR =1.072; 95% CI: 1.008–1.141; P=0.03), with a similar trend noted in PD-L1-negative patients (HR =0.313; 95% CI: 0.941–1.833; P=0.11), former smokers (HR =1.098; 95% CI: 1.029–1.172; P=0.005), and those with non-squamous NSCLC (HR =1.084; 95% CI: 1.017–1.155; P=0.03). It is noteworthy that these associations were only evident for NLR and not dNLR. Interestingly, elevated ANC was consistently and negatively associated with poorer PFS in the same subgroups as NLR, including those treated with ICI monotherapy.
In summary, NLR or ANC as a predictive factor for PFS was globally consistent across all subgroups. However, NLR and ANC appeared to be more significant and relevant in selected subgroups such as older patients over 65 years old, former smokers, non-squamous NSCLC, or those with a ECOG-PS 0–1. Conversely, the efficacy of dNLR to predict PFS was notably lower in the overall population, prompting subgroup analysis to explore potential relevance limited to specific subgroups. Thus, dNLR was effective in predicting PFS in only two conditions: patients older than 65 years or with a ECOG-PS <2.
dNLR, ANC and NLR comparisons for OS
As both NLR, ANC and dNLR were significantly predictive in terms of OS in overall population, we conducted the same subgroups comparisons. Survival analysis demonstrated that the association between NLR or dNLR and OS found in the overall population seemed to be observed in patients exposed to ICI alone with P=0.01 for NLR (HR =1.080 with 95% CI: 1.017–1.146) and P=0.051 for dNLR (HR =1.241 with 95% CI: 0.999–1.542), while there was no significant association with chemotherapy combination for either NLR (HR =1.089 with 95% CI: 0.975–1.215; P=0.13) or dNLR (HR =1.300 with 95% CI: 0.919–1.839; P=0.14) (Figure 5). However, subgroup analyses did not reach statistical significance for the ANC levels. As observed for PFS, NLR, ANC and dNLR were associated with OS in patients aged ≥65 years or in good general condition (ECOG-PS 0–1) (Table 6).

Table 6
| Subgroups | NLR | dNLR | ANC | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age | ||||||||
| ≥65 years | 1.124 (1.050–1.203) | 0.001 | 1.513 (1.137–2.012) | 0.004 | 1.064 (1.026–1.104) | 0.001 | ||
| <65 years | 1.026 (0.899–1.171) | 0.71 | 1.085 (0.835–1.409) | 0.54 | 1.084 (1.003–1.171) | 0.04 | ||
| Smoking history | ||||||||
| Current smokers | 1.183 (0.934–1.498) | 0.16 | 2.321 (1.066–5.068) | 0.03 | 1.170 (1.035–1.322) | 0.01 | ||
| Former smokers | 1.108 (1.042–1.178) | 0.001 | 1.217 (0.991–1.494) | 0.06 | 1.062 (1.026–1.099) | 0.001 | ||
| PD-L1 expression (TPS) | ||||||||
| Negative (TPS <1%) | 1.315 (0.920–1.879) | 0.13 | 1.742 (0.744–4.078) | 0.20 | 1.232 (1.008–1.505) | 0.04 | ||
| Positive (TPS ≥1%) | 1.092 (1.031–1.157) | 0.003 | 1.215 (0.973–1.519) | 0.09 | 1.058 (1.023–1.095) | 0.001 | ||
| Histological sub type | ||||||||
| Non-Sq NSCLC | 1.096 (1.032–1.164) | 0.003 | 1.166 (0.944–1.439) | 0.15 | 1.065 (1.031–1.099) | 0.001 | ||
| Sq-NSCLC | 1.501 (0.964–2.336) | 0.07 | 2.049 (0.968–4.336) | 0.06 | 1.586 (0.984–2.556) | 0.057 | ||
| Regimen | ||||||||
| ICI alone | 1.080 (1.017–1.146) | 0.01 | 1.241 (0.999–1.542) | 0.051 | 1.047 (1.010–1.085) | 0.01 | ||
| CT-ICI | 1.089 (0.975–1.215) | 0.13 | 1.300 (0.919–1.839) | 0.13 | 1.100 (1.035–1.170) | 0.001 | ||
| ECOG-PS | ||||||||
| 0–1 | 1.108 (1.042–1.178) | 0.001 | 1.272 (1.045–1.547) | 0.02 | 1.072 (1.034–1.112) | 0.001 | ||
| ≥2 | 1.083 (0.926–1.268) | 0.32 | 1.022 (0.574–1.820) | 0.94 | 1.062 (0.991–1.139) | 0.09 | ||
P value was considered significant if <0.05. ANC, absolute neutrophil count; CI, confidence interval; CT-ICI, chemo-immunotherapy; dNLR, derived neutrophil-to-lymphocyte ratio; HR, hazard ratio; ICI, immune checkpoint inhibitor; NLR, neutrophil-to-lymphocyte ratio; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1; ECOG-PS, Eastern Cooperative Oncology Group-performance status; Sq, squamous cell carcinoma; TPS, tumor proportion score.
We noted that elevated NLR was also associated with shorter OS in the subgroup of former smokers (HR =1.108 with 95% CI: 1.042–1.178; P=0.001) while dNLR ≥2.5 showed a worse OS in the subgroup of current smokers (HR =2.321 with 95% CI: 1.066–5.068; P=0.03). The association between ANC and OS did not appear to be influenced by smoking status. An increased NLR ratio was also associated with worse OS in PD-L1-positive patients (HR =1.092 with 95% CI: 1.031–1.157; P=0.003), in non-squamous NSCLC (HR =1.096 with 95% CI: 1.032–1.164; P=0.003) with a statistical trend in squamous NSCLC (HR =1.501 with 95% CI: 0.964–2.336; P=0.07).
In summary, NLR globally predicted OS across all subgroups of interest, and particularly in older patients, former smokers, PD-L1-positive individuals, and those in good general condition. Similar results were observed for dNLR and ANC, although with weaker significances for similar sample sizes.
Discussion
Key findings
Our retrospective, single-center study investigated NLR, ANC and dNLR in 70 consecutive patients diagnosed with metastatic NSCLC, where frontline treatment consisted of either single-agent immunotherapy or platinum-based combination chemotherapy. Consistent with existing literature, our findings revealed that elevated pre-treatment NLR, ANC or dNLR levels were associated with decreased survival. NLR has been extensively studied as a prognostic factor (22,23), with meta-analyses results suggesting a potential predictive effect in NSCLC (14) or melanoma (24). In NSCLC, Wang et al. conducted a meta-analysis in 2019, analyzing 27 articles and over 4,000 patients (14). The results demonstrated that elevated baseline NLR was associated with decreased survival, particularly in terms of PFS, with an HR of 1.45 (95% CI: 1.28–1.66). Conversely, Yang et al. (10) proposed dNLR as an alternative predictive factor, hypothesizing that it might better reflect systemic inflammation than NLR by encompassing all WBCs, including monocytes and other granulocytes involved in the early systemic inflammatory response. Eight studies were pooled in Yang et al.’s meta-analysis, indicating that higher dNLR significantly predicted poor PFS, with an HR of 1.38 (95% CI: 1.23–1.55). Concerning ANC impact to predict clinical outcomes for NSCLC patients treated by ICI, Pu et al. reported similar results with worse objective response rate (ORR), PFS, and OS for patients with baseline elevated ANC ≥7.5 (25). Very interestingly, concordant results for both ANC, NLR and dNLR were observed in others solid cancer type such as melanoma, reporting poorer clinical outcomes in case of elevated of inflammation markers (26). However, as previously noted, no direct comparison was made between NLR, ANC and dNLR.
Strengths and limitations
It is important to acknowledge several limitations in our study, primarily stemming from its retrospective and single-center design. The lack of significance observed for dNLR or ANC compared to NLR may be attributed to a potential lack of statistical power, particularly considering the absence of clinical associations between ECOG or PD-L1 and PFS or OS in this analysis. However, it is worth noting that our study represents the first dimensionally sized cohort to compare NLR and dNLR in the context of first-line immunotherapy, either alone or in combination with chemotherapy. Future investigations should delve deeper into the interactions between these biomarkers and other clinical variables to enhance their utility in clinical practice, including immune related adverse events (irAEs). Primarily due to the retrospective nature of our study, which makes the evaluation of irAEs challenging, we did not investigate the potential association between irAEs and inflammatory markers. However, previous publications have reported associations between a low NLR (NLR <3) and an increased risk of developing irAEs. For instance, a meta-analysis including 7 studies with 1,068 cancer patients demonstrated that a low NLR [odds ratio (OR) =3.02; 95% CI: 1.51–6.05; P=0.002] and a low platelet-to-lymphocyte ratio (L-PLR) (OR =1.83; 95% CI: 1.21–2.76; P=0.004) were significantly associated with irAEs. Specifically, in subgroup analyses, an NLR cut-off of 3 was significantly correlated with irAEs (OR =2.63; 95% CI: 1.63–4.26; P<0.001), suggesting that inflammatory markers may help identify patients at higher risk for irAEs, warranting closer monitoring (27). The validation of these parameters holds substantial promise, as they present the opportunity for a cost-effective, non-invasive, and easily interpretable test that could aid in determining patients’ status, prognosis, and response to therapeutic interventions.
Comparison with similar researches
Only a small number of studies have examined these ratios in patients undergoing chemo-immunotherapy, which remains the recommended combination for most of our patients, particularly as a first-line treatment when PD-L1 expression is ≤49%. To the best of our knowledge, only two studies evaluated the utility of these circulating markers in the context of chemotherapy and immunotherapy combination. Chen et al. (28) published in 2023 a retrospective study with 142 stage IV patients (PD-L1 ≤49%) and found in subgroup analyse an advantage to chemoimmunotherapy combination (n=28): a low NLR level (<3.6) was predictive of better PFS and OS only in the chemotherapy combined with immunotherapy group, which was not observed in the chemotherapy alone group (P=0.001 and P=0.005, respectively). Consistently, Banna et al. (29) observed in a cohort of 308 metastatic NSCLC patients treated with chemoimmunotherapy that higher NLR levels (>4) were associated with shorter OS (median 11.8 vs. 14.9 months, P=0.02) and PFS (median 6.6 vs. 9.0 months, P=0.018). Petrova et al. (30) conducted one of the limited number of studies that explored NLR by comparing subgroups receiving immunotherapy and chemotherapy. In this study, NLR showed a significant association with OS in both patients treated with chemotherapy (HR =8.56; 95% CI: 4.34–16.93; P<0.001) or immunotherapy alone (HR =7.94; 95% CI: 3.99–15.78; P<0.001), using a consistent cutoff value of 5.0. All of this suggests that NLR could be an overall prognostic factor not specific to immunotherapy, as its superiority in the context of either immunotherapy or chemotherapy has yet to be demonstrated.
Explanations of findings
To our knowledge, this is the first study to suggest a potentially greater relevance of NLR over dNLR in patients undergoing immunotherapy for NSCLC. Specifically, NLR appeared to exhibit a more pronounced predictive effect, particularly in the population treated solely with immunotherapy. The observed effect in the overall population seemed to be driven by the outcomes observed in the immunotherapy subgroup, prompting speculation about a potential reduction in the predictive capacity of NLR in the context of immunotherapy combined with chemotherapy. This observed difference may be attributed to the additive or synergistic effects of combining immunotherapy and chemotherapy, which represent distinct active substances that collectively influence the tumor immune response. Such synergistic effects have been previously demonstrated; for instance, results from the KEYNOTE-189 trial (31) indicated that combining chemotherapy with pembrolizumab resulted in a significant improvement in OS and PFS, regardless of PD-L1 expression levels. This suggests that the predictive efficacy of PD-L1 in anticipating tumor response may diminish when immunotherapy is combined with chemotherapy.
The lack of association between NLR, ANC, dNLR, and the evaluated clinical factors, including age, PD-L1 expression, and ECOG-PS, suggests that these biomarkers operate independently of the clinical characteristics of NSCLC patients. These findings are consistent with some previous retrospective studies (10,11,14,15), although data from literature are not uniform on this matter, as results of multivariate analyses vary across studies due to the often-heterogeneous populations undergoing immunotherapy and the statistical methods employed. It is noteworthy that the NLR cut-off of 5 identified in our study aligns with commonly reported thresholds in the literature (19), validating its utility. However, the dNLR cut-off of 2.5 did not exhibit the same consistency with previous findings, indicating variability in the predictive value of this biomarker across different study populations and highlighting a lack of reproducibility. Consequently, NLR appears to be a more consistent biomarker with less variability than dNLR in our study. Additionally, we observed that NLR adhered to a linearity assumption, showing an effect inversely proportional to OS, which was not demonstrated for dNLR and ANC. All these data support the superior predictive capacity of NLR but complicates the prediction of responder vs. non-responder profiles in real-life scenarios in the absence of a clear and robust threshold effect.
Implications and actions needed
As indicated by our results, NLR emerges as a comprehensive prognostic factor, demonstrating predictive efficacy for both OS and PFS, notably within the subset of patients receiving immunotherapy alone. One of the limitations of our study is the absence of a chemotherapy-treated control group, which would have helped clarify whether the predictive capabilities of NLR are specific to immunotherapy or not. Additionally, NLR could be considered in combination with other relevant predictive parameters, as suggested by Valero et al., who observed an enhanced predictive capacity when combined with TMB (32).
Conclusions
In this study, NLR, ANC and dNLR were compared for the first time in NSCLC patients undergoing first-line immunotherapy or chemo-immunotherapy. NLR emerged as a superior prognostic indicator, with a cut-off of 5, consistent with existing literature, which may also serve as an independent predictive factor, particularly in patients treated with immunotherapy alone. Further clinical studies are needed to fully assess the utility of these biomarkers, potentially in conjunction with other clinical or biological parameters to allow better patient selection and optimal clinical management.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-808/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-808/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-808/prf
Funding: This study was supported in part by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-808/coif). G.D. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Chiesi, Sanofi, GSK (personal fees), outside of the submitted work. J.A. reports grants from AMGEN, French Innovative Research Fund; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Roche, Pfizer, MSD, Bristol-Myers Squibb, Novartis, AstraZeneca, Takeda, Sanofi, and Amgen; support for attending meetings and/or travel from Roche, Pfizer, MSD, Takeda, and Sanofi. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional committee board of University Hospital of Reims’ data protection departments (No. MR004160420221). In accordance with French law, an information notice was sent to all patients to fulfill the requirements for non-opposition.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Assi HI, Kamphorst AO, Moukalled NM, et al. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 2018;124:248-61. [Crossref] [PubMed]
- Hendriks LE, Kerr KM, Menis J, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:358-76. [Crossref] [PubMed]
- Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020;126:260-70. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Borghaei H, Gettinger S, Vokes EE, et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021;39:723-33. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. [Crossref] [PubMed]
- Yang T, Hao L, Yang X, et al. Prognostic value of derived neutrophil-to-lymphocyte ratio (dNLR) in patients with non-small cell lung cancer receiving immune checkpoint inhibitors: a meta-analysis. BMJ Open 2021;11:e049123. [Crossref] [PubMed]
- Zhang Q, Gong X, Sun L, et al. The Predictive Value of Pretreatment Lactate Dehydrogenase and Derived Neutrophil-to-Lymphocyte Ratio in Advanced Non-Small Cell Lung Cancer Patients Treated With PD-1/PD-L1 Inhibitors: A Meta-Analysis. Front Oncol 2022;12:791496. [Crossref] [PubMed]
- Murakami Y, Tamiya A, Taniguchi Y, et al. Retrospective analysis of long-term survival factors in patients with advanced non-small cell lung cancer treated with nivolumab. Thorac Cancer 2022;13:593-601. [Crossref] [PubMed]
- Sibille A, Henket M, Corhay JL, et al. White Blood Cells in Patients Treated with Programmed Cell Death-1 Inhibitors for Non-small Cell Lung Cancer. Lung 2021;199:549-57. [Crossref] [PubMed]
- Wang Z, Zhan P, Lv Y, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res 2019;8:214-26. [Crossref] [PubMed]
- Huang W, Luo J, Wen J, et al. The Relationship Between Systemic Immune Inflammatory Index and Prognosis of Patients With Non-Small Cell Lung Cancer: A Meta-Analysis and Systematic Review. Front Surg 2022;9:898304. [Crossref] [PubMed]
- Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:351-7. [Crossref] [PubMed]
- Navani V, Meyers DE, Ruan Y, et al. Lung Immune Therapy Evaluation (LITE) Risk, a Novel Prognostic Model for Patients With Advanced Non-Small Cell Lung Cancer Treated With Immune Checkpoint Blockade. Clin Lung Cancer 2023;24:e152-9. [Crossref] [PubMed]
- Banna GL, Cortellini A, Cortinovis DL, et al. The lung immuno-oncology prognostic score (LIPS-3): a prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer. ESMO Open 2021;6:100078. [Crossref] [PubMed]
- Ancel J, Dormoy V, Raby BN, et al. Soluble biomarkers to predict clinical outcomes in non-small cell lung cancer treated by immune checkpoints inhibitors. Front Immunol 2023;14:1171649. [Crossref] [PubMed]
- Wang H, Yang R, Liu D, et al. Association of pretreatment neutrophil-to-lymphocyte ratio with clinical outcomes in cancer immunotherapy: An evidence synthesis from 30 meta-analyses. Int Immunopharmacol 2024;132:111936. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Bi H, Ren D, Xiao Y, et al. Prognostic implications of neutrophil-to-lymphocyte ratio in patients with extensive-stage small cell lung cancer receiving chemoimmunotherapy: A multicenter, real-world study. Thorac Cancer 2024;15:559-69. [Crossref] [PubMed]
- Mosca M, Nigro MC, Pagani R, et al. Neutrophil-to-Lymphocyte Ratio (NLR) in NSCLC, Gastrointestinal, and Other Solid Tumors: Immunotherapy and Beyond. Biomolecules 2023;13:1803. [Crossref] [PubMed]
- Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther 2018;11:955-65. [Crossref] [PubMed]
- Pu D, Xu Q, Zhou LY, et al. Inflammation-nutritional markers of peripheral blood could predict survival in advanced non-small-cell lung cancer patients treated with PD-1 inhibitors. Thorac Cancer 2021;12:2914-23. [Crossref] [PubMed]
- Ou Y, Liang S, Gao Q, et al. Prognostic value of inflammatory markers NLR, PLR, LMR, dNLR, ANC in melanoma patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review. Front Immunol 2024;15:1482746. [Crossref] [PubMed]
- Lu HR, Zhu PF, Deng YY, et al. Predictive value of NLR and PLR for immune-related adverse events: a systematic review and meta-analysis. Clin Transl Oncol 2024;26:1106-16. [Crossref] [PubMed]
- Chen CW, Lin CY, Tsai JS, et al. Low neutrophil-to-lymphocyte ratio predicts overall survival benefit in advanced NSCLC patients with low PD-L1 expression and receiving chemoimmunotherapy. Front Oncol 2023;13:1238876. [Crossref] [PubMed]
- Banna GL, Cantale O, Muthuramalingam S, et al. Efficacy outcomes and prognostic factors from real-world patients with advanced non-small-cell lung cancer treated with first-line chemoimmunotherapy: The Spinnaker retrospective study. Int Immunopharmacol 2022;110:108985. [Crossref] [PubMed]
- Petrova MP, Eneva MI, Arabadjiev JI, et al. Neutrophil to lymphocyte ratio as a potential predictive marker for treatment with pembrolizumab as a second line treatment in patients with non-small cell lung cancer. Biosci Trends 2020;14:48-55. [Crossref] [PubMed]
- Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 2023;41:1992-8. [Crossref] [PubMed]
- Valero C, Lee M, Hoen D, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun 2021;12:729. [Crossref] [PubMed]


