
FOXO3a regulation of non-small cell lung cancer radiotherapy resistance through the PINK1/Parkin pathway of protective mitophagy
Highlight box
Key findings
• We first demonstrated that PINK1/Parkin-mediated mitochondrial autophagy positively correlated with the radioresistance in a non-small cell cancer (NSCLC) cell line and confirmed that FOXO3a played a significant role in regulating NSCLC cell radioresistance through this pathway.
What is known and what is new?
• Previous studies have identified a limited role for FOXO3a in modulating tumor cell sensitivity and resistance to radiotherapy or chemotherapy. The PINK1/Parkin pathway, a well-established mitochondrial autophagy pathway, has been studied in relation to tumor cell radiosensitivity and resistance; yet, the comprehensive role of the FOXO3a–PINK1/Parkin pathway in NSCLC remains unexplored.
• This is the first study to investigate FOXO3a’s regulatory role in NSCLC radiotherapy resistance using a NSCLC radiotherapy resistance model. Our findings revealed, for the first time, that FOXO3a significantly influenced NSCLC radioresistance via PINK1/Parkin-mediated mitochondrial autophagy.
What is the implication, and what should change now?
• Using the radiotherapy resistance model, we confirmed the role of the FOXO3a–PINK1/Parkin pathway in promoting NSCLC radioresistance. This finding provides a theoretical and experimental foundation for identifying new molecular targets to overcome NSCLC radioresistance and suggests novel approaches for clinical intervention and treatment.
• We will investigate the complex mechanisms by which FOXO3a influences the PINK1/Parkin pathway, including phosphorylation, acetylation, ubiquitination, and intermediate target genes or cytokines. Additionally, designing and screening specific inhibitors that target the FOXO3a gene and translating these findings into clinical applications will be key focuses of our future research.
Introduction
Lung cancer exhibits the highest morbidity and mortality rates among all malignancies, both in China and globally, with non-small cell lung cancer (NSCLC) constituting approximately 80% of cases. Lung adenocarcinoma and lung squamous cell carcinoma (LUSC) are the most common histological types of NSCLC (1,2). More than 50% of NSCLC cases are initially diagnosed as locally or distantly metastatic and incurable diseases. Radiation therapy is an important and effective approach for the treatment of locally advanced and metastatic NSCLC as sometimes more definitive and commonly, and it is also an essential component of combined treatment modalities. However, distinct mechanisms induced by radiation can lead to variable degrees of radioresistance in malignant tumors, resulting in treatment failure, tumor recurrence and metastasis, and poor prognosis (3-5). Investigations into the underlying causes and regulatory mechanisms of tumor radioresistance remain an active area of research, garnering increasing scientific interest.
Autophagy plays a pivotal role in the elimination, degradation, and recycling of radiation-damaged intracellular organelles and macromolecules. Specifically, ionizing radiation can specifically trigger the selective autophagy process in tumor cells, in which dysfunctional and damaged mitochondria can be effectively degraded, eliminated, and reused through autophagy mechanisms. As a spontaneous mechanism for clearing damaged mitochondria after radiotherapy, mitophagy helps tumor cells adapt to and cope with their environment (6,7). However, the specific role of mitophagy in tumorigenesis and tumor progression remains poorly understood (8). Emerging evidence suggests that the failure of mitophagy may exacerbate oncogenic stress and promote malignant transformation (9-11). The molecular mechanisms regulating mitophagy are complex and diverse often involving crosstalk between pathways. Among these, the PINK1/Parkin pathway is the most well-characterized pathway for mitophagy (12). Many studies have found that Parkin promotes oncogenesis and in comparison with normal tissues, lung cancer (13) and esophageal cancer (14) tissues show upregulation of PINK1, which can promote tumor cell proliferation and chemotherapy resistance. Silencing of PINK1 can not only inhibit the proliferation and migration of lung cancer cells but also induce apoptosis (13). Tumor cells can impact the efficacy of radiotherapy through mitophagy under radiation stress (15); therefore, elucidating the interplay between mitophagy and radiotherapy resistance may represent a significant step forward in developing novel anticancer treatment strategies. Forkhead box O 3a (FOXO3a), the most active member of the FOXO family, has garnered significant research attention in recent years. Accumulating evidences suggest that FOXO3a, as an essential transcription factor for cell survival, not only regulates various cellular functions but also serves as a key regulatory factor in cancer progression, participating in multiple physiological and pathological processes such as tumor proliferation, differentiation, invasion, metastasis, and cell death (16-19). Research has found that FOXO3a has a certain influence on the radiotherapy and chemotherapy resistance of tumor cells (20-22), however there is no consensus on the role of FOXO3a in the radiotherapy resistance of NSCLC tumor cells. Additionally, while it has been reported that FOXO3a participates in receptor-mediated mitophagy, such as that of BNIP3 and FUNDC1 (23,24), the role of FOXO3a in PINK1/Parkin-mediated mitophagy remains unclear. In this study, we investigated the regulatory role of the FOXO3a gene in the radiotherapy resistance of NSCLC cells and the effect of FOXO3a on the mitophagy mediated by the PINK1/Parkin pathway in the radiotherapy resistance of NSCLC cells. Our findings provide new insights and novel therapeutic approaches for improving radiotherapy resistance in NSCLC and provide a theoretical and experimental foundation for developing targeted molecular therapies to overcome radioresistance in clinic. We present this article in accordance with the ARRIVE and MDAR reporting checklists (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-181/rc).
Methods
Cell culture and irradiation
Human NSCLC cell lines (human lung adenocarcinoma cell line A549 and human LUSC cell line H520) were purchased from the Shanghai Institute of Cell Biology (Shanghai, China). Cell lines were grown in Dulbecco’s Modified Eagle Medium (DMEM) with high-glucose (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS) (Nanjing Vazyme Biotech Co., Nanjing, China) and 1% streptomycin, cultured in a 37 °C incubator with 5% CO2.
To generate radioresistant cell lines, A549 and H520 cells were irradiated with fractionated doses of X-rays (Varian Medical Systems, Palo Alto, CA, USA) at 2 Gy per fraction (≥60 Gy in total). The radiation field was 10 cm × 10 cm, and the source-to-target distance was 100 cm. The cells were passaged two or three times after each irradiation so that they had sufficient vitality for the subsequent irradiation. After fractionated irradiation of ≥60 Gy, surviving cells that became more radioresistant than the parent cells were labelled as A549/X and H520/X cells, respectively.
Colony formation assay
Depending on the planned radiation dose, cells were planted in six-well plates at different densities and incubated for about 12 h. Following irradiation with different doses of X-rays and a culture of 10–14 days until colonies contained more than 50 cells, the plates were washed once with phosphate-buffered saline (PBS). Subsequently, the cell colonies were fixed with 4% paraformaldehyde for 20 min, stained with crystal violet for 30 min, and counted after a washing. The survival fraction and survival curve of cells were calculated and plotted using GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA) as previously described (25).
Cell wound-healing assay
Wound-healing assays were used to evaluate the migration capacity of cells. Approximately 2×105 cells were plated in six-well plates, and wounds were created via scratching with 10-µL pipettes tip when the cell confluence reached about 90%. A culture medium containing 1% FBS was added to the six-well plates after media were aspirated and the separated cells were washed with PBS. The six-well plates were gently moved onto a microscope, and the cell scratch state at 0 h was recorded as a control. Cells were treated with or without radiotherapy, and the same field was photographed again every 24 h. At least three fields were observed in each independent experiment, and the scratch healing ability of cells in each group was compared.
Transwell invasion assay
Transwell invasion assays were used to evaluate the invasion capacity of cells. A polymerization membrane was formed over a Transwell chamber (Corning, 8 µm) with Matrigel (Corning, 354248, NY, USA). The cells were seeded into the upper chamber with serum-free medium {[3–4]×104 cells}, and complete culture medium containing 10% FBS was used as the culture medium in the bottom of the chamber. After 6–8 h of cell adhesion and experimental treatment, the culture plates were incubated in an incubator for 18–36 h. The cells were then fixed and stained with crystal violet, and the invaded cells were counted under an inverted light microscope. The number of invaded cells was quantified by counting the number of cells from 3–5 random fields at 400×magnification.
Protein extraction and western blotting
Radioimmunoprecipitation (RIPA) lysis buffer (Beyotime Biotechnology, Nantong, China) was used to extract total protein from the samples at the indicated time points following the designated experimental treatments, and the protein concentration was determined using a bicinchoninic acid protein assay kit (Beyotime Biotechnology). The protein samples (20 µg/lane) were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Epizyme, Cambridge, MA, USA) and were then transferred to nitrocellulose membranes. After blocking in 5% nonfat milk was completed, the membranes were incubated with the primary antibodies at 4 °C for 12–14 h and then incubated with anti-rabbit secondary antibody at room temperature for 2 h. Enhanced chemiluminescence (ECL) was performed with an ECL Western blotting detection kit (Thermo Fisher Scientific, Waltham, MA, USA), and protein bands were analyzed using Bio-Rad ChemiDoc (Bio-Rad, Hercules, CA, USA). GAPDH was used as the internal reference. The antibodies are listed in Table S1.
RNA isolation and real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using SteadyPure rapid RNA extraction kit (Accurate Biology, China), and the concentration was quantified. Following this, 500 ng of total RNA was reversely transcribed into complement DNA (cDNA) using EvoM-MLV reverse transcription reagent premix solution (Accurate). Subsequently, the SYBR Green Pro Taq HS Premix qPCR Kit (Accurate) was used to conduct RT-qPCR. Each RNA sample was run in three independent experiments. The forward and reverse primer sequences are listed in Table S2.
High-throughput transcriptomics
We examined the mitophagy-related differential genes associated with radiotherapy resistance by performing high-throughput transcriptome sequencing of the NSCLC parental cell lines A549 and H520 and the corresponding radioresistant cells A549/X and H520/X. The parental and radioresistant cells were laid in two parts (with subsequent radiation treatment/without radiation treatment) with three biological replicates each and cultured to the logarithmic growth phase. One of the cell lines received a 4-Gy dose of X-ray radiotherapy, and the other cell line was not treated with radiotherapy. Total RNA was extracted from cells within 1–2 h after radiation for testing in high-throughput transcriptome sequencing analysis. The high-throughput transcriptome sequencing of this study was assisted by Tiangen Biochemical Technology (Beijing, China).
Transmission electron microscopy (TEM)
The ultrastructural analysis of mitochondrial autophagosomes was carried out via TEM. Cells were collected and fixed in electron mirror fixative and fixed in 1% osmium tetroxide solution. After dehydration was completed with absolute ethanol gradient, the samples were permeabilized with acetone and embedding agent. Samples were then embedded, polymerized, cut into 60- to 80-nM ultrathin sections, and double stained with uranium and lead. Finally, the mitochondrial autophagosomes in the cells were observed by TEM.
Transfection with transient small interfering RNA
The small interfering RNAs (siRNAs) of FOXO3a were designed and provided by OBiO (Shanghai, China). According to the different sequences, siRNAs were labeled as siRNA#1, siRNA#2, siRNA#3, siNC-negative, and siNC-positive. The siRNA constructs are listed in Table S3. Stock solutions of siRNAs and oligonucleotides were made at 20 µM in RNase-free water and stored at –20 °C. Cells were transfected using Lipofectamine 2000 reagent (Thermo Fisher Scientific) according to the instructions, and the effect of transfection was evaluated by Western blotting.
Confocal microscopy observation of COX8-EGFP-mCherry fluorescence
To examine the fusion of mitochondria and lysosomes, we transfected COX8-EGFP-mCherry fluorescent reporter plasmids (OBiO, Shanghai, China) into parental cell lines A549 and H520, as well as into their corresponding radioresistant cell lines A549/X and H520/X. After transfection with this plasmid, mitochondria typically display both red and green fluorescence, which overlap to produce an orange-yellow fluorescence. However, when mitochondria are engulfed by autophagosomes to form mitophagolysosomes, the mitochondria carrying fluorescent plasmids are located within acidic lysosomes. The green fluorescence is sensitive to low pH environments and quenches, resulting in the fluorescent plasmids only exhibiting red fluorescence. Therefore, the number of red dots within cells can be used for quantitative measurement of the strength of mitophagy occurrence. Cells with a stable expression of the COX8-EGFP-mCherry plasmid were constructed by using the optimal multiplicity of infection (MOI) as indicated by pre-experiments, and the amount of virus was determined. After experimental treatment, the cells were fixed, permeated, and sealed under a laser confocal microscope for observation and photography. The number of red points in the cells was used for quantitative measurement of the intensity of mitochondrial autophagy.
Vectors, transfection, and retroviral infection
The FOXO3a short hairpin (FOXO3a-sh) plasmids of three sequences from the DNA library of Shanghai Jiao Tong University School of Medicine were selected for this study. The plasmids were extracted and transfected into HEK293T cells to obtain the viral solution. Subsequently, NSCLC radiation-resistant cells A549/X and H520/X were infected with viral solution and then screened with puromycin-containing media for several generations to obtain stably transfected cells. The knockdown efficiency of the FOXO3a gene was evaluated by Western blotting assays. The sequences of the short hairpin RNA (shRNA) against FOXO3a are provided in Table S4.
Nude mouse xenograft models and irradiation
Three-to-four-week-old BALB/c nude mice (BALB/c nude is an immunodeficient strain derived from the standard BALB/c mice, characterized by a Foxn1 gene mutation that prevents thymus and T cell development, n=40) (Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) were randomly divided into four groups. Four transfected NSCLC radiotherapy-resistant cell lines (A549/X-NC, A549/X-sh3, H520/X-NC, and H520/X-sh3) were injected subcutaneously into the right back of the nude mice. When the tumors increased to 100 mm3, the tumor-bearing mice were administered 2 Gy of the fractionated radiotherapy per day for 3 consecutive days, while the control group did not receive any X-ray radiotherapy. The experiment was ended 14 days after radiotherapy. The longest dimension (L) and shortest dimension (W) of tumor were measured every two days with a digital caliper, and the volume of tumor was calculated according to the following formula: tumor volume (mm3) = L×W2/2.
Analysis of human gene/data from The Cancer Genome Atlas (TCGA) database
We evaluated the prognostic significance of elevated FOXO3a-PINK1/Parkin pathway expression in NSCLC patients using data from TCGA. Survival analyses included overall survival (OS), disease-specific survival (DSS), and progression-free interval (PFI).
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Animal experiments were performed under a project license (No. K24-206Y) approved by Institutional Animal Care and Use Committee of Shanghai Pulmonary Hospital, in compliance with the Shanghai Pulmonary Hospital guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 8.0, and all experiments were repeated three times. Quantitative values were presented as the mean ± standard deviation (SD). A Student t-test was used for comparisons between two groups. For all analyses, a P value less than 0.05 was considered statistically significant.
Results
Development of radioresistant NSCLC cell lines
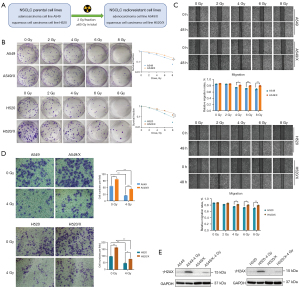
We employed a medical linear accelerator to simulate clinical radiotherapy conditions and deliver conventional fractionation of X-ray irradiation on cells through multifractionated daily low-dose exposures. Through continuous cell culture, digestion, and passage, we gradually eliminated cells that had undergone lethal damage or that had failed to repair potentially lethal damage through a series of induction and screening processes. Ultimately, NSCLC cells with stable conditions and radiation-resistant properties (A549/X and H520/X) were successfully obtained (Figure 1A). We found that the radiation tolerance and cell proliferation ability of A549/X and H520/X radiotherapy-resistant cells were significantly enhanced compared to those of the parent cells through clone formation experiments (Figure 1B). The wound-healing assay revealed that the migration ability of the radioresistant cells was significantly enhanced compared to that of the parent cells (Figure 1C). The Transwell invasion assays demonstrated that regardless of the presence or absence of irradiation, A549/X and H520/X radioresistant cells migrated from the upper chamber to the lower chamber of the Transwell with significantly higher numbers than the parent cells did, indicating an enhanced cell invasion ability of the radioresistant cells (Figure 1D). γH2AX, a well-established biomarker of DNA double-strand breaks, was employed to evaluate the sensitivity or resistance of tumor cells to radiotherapy stress. In this experiment, comparative analysis of γH2AX expression levels in both radioresistant and parental NSCLC cell lines demonstrated significantly attenuated DNA damage response in resistant populations following X-ray exposure, indicating that the ability of resistant cells to defend against X-ray-induced DNA damage was significantly stronger than that of the parental cell lines (Figure 1E).
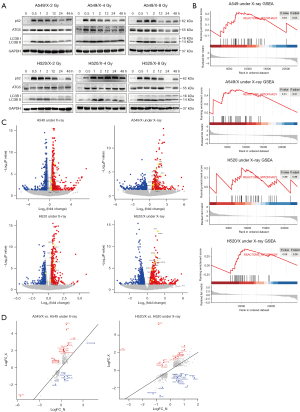
High-throughput transcriptomic analysis of mitochondrial autophagy-related genes with enriched expression in radioresistant cell lines
We employed A549/X and H520/X radiotherapy-resistant cells to identify the optimal detection timepoint and radiation dose for autophagy response following irradiation. Western blotting assays were conducted to detect the expression levels of the autophagy-related proteins (LC3, Atg5, and p62) in these two cell lines. The results indicated that autophagy activation was rapidly induced within 0.5 hours post-irradiation and sustained at elevated levels throughout the 0.5–2.0 hours observation window. Notably, the impact of increasing or decreasing radiation doses on autophagy was not significant, and even a low dose of radiation could significantly enhance the autophagy level in NSCLC cells. Based on these comprehensive findings, we established 4 Gy as the standard radiation dose for subsequent autophagy studies, with a 1–2-hour post-irradiation window identified as the optimal temporal frame for autophagy assessment (Figure 2A). To elucidate the molecular mechanisms underlying radioresistance in NSCLC, we performed high-throughput RNA sequencing on both parental (A549 and H520) and their corresponding radioresistant (A549/X and H520/X) cell lines under both irradiated (4 Gy X-ray) and non-irradiated conditions. This comprehensive transcriptomic analysis aimed to identify differentially expressed genes (DEGs) associated with acquired radioresistance. Gene set enrichment analysis (GSEA) revealed enrichment of mitophagy-related pathways in radioresistant cell lines following 4 Gy irradiation compared to their parental counterparts. Notably, A549/X cells demonstrated particularly pronounced differential enrichment of mitophagy-associated genes (Figure 2B). This suggests that the characteristic radioresistance in the NSCLC radioresistant cell lines may be associated with the enhanced response of mitochondrial autophagy. Subsequent targeted analysis of DEGs within the mitophagy-related pathways revealed consistent upregulation of FOXO3a transcript levels in both radioresistant cell lines (A549/X and H520/X) compared to their parental counterparts (Figure 2C). Moreover, focused comparative analysis of differentially expressed mitophagy-associated genes demonstrated significant upregulation of FOXO3a expression in both radioresistant cell variants (A549/X and H520/X) relative to their parental counterparts (Figure 2D). Experiments and analytical results indicated that the radioresistance characteristics of NSCLC radioresistant cell lines were associated with the upregulated response of the FOXO3a gene. In addition, we found that the patients with lung adenocarcinoma and higher expression levels of FOXO3a gene had a shorter OS and DSS. Furthermore, the PFI was worse with a log-rank statistic of P=0.03 [hazard ratio (HR) =1.36, 95% confidence interval (CI): 1.03–1.80] (Figure S1A). Thus, the high expression of FOXO3a gene may be one of the adverse factors restricting the survival and prognosis of patients with lung adenocarcinoma. The radioresistance characteristics of radiotherapy-resistant NSCLC cells are likely related to the enhancement of the upregulation of FOXO3a gene reactivity and the enrichment of mitochondrial autophagy-related genes in resistant cells after radiotherapy, and we speculate that FOXO3a may be a key gene in the regulation of radioresistance in NSCLC.
Upregulation of the mitochondrial autophagy PINK1/Parkin pathway and the FOXO3a gene after radiotherapy
We measured the expression levels of autophagy-related proteins using Western blot assays in both parent cell lines and radioresistant cell lines with or without radiotherapy. The results showed that, compared with those of parent cell lines, the baseline expression levels of mitochondrial autophagy-related proteins in radioresistant cell lines were significantly higher. After radiation exposure, the expression levels of mitochondrial autophagy-related proteins in radioresistant cell lines were significantly increased (Figure 3A). When observing the mitochondrial autophagosomes in cells under TEM, we found that compared with parent cells, the radioresistant cells exhibited increase in the number of mitochondrial autophagic vacuoles. After radiation exposure, the increase in the number of mitochondrial autophagic vacuoles in radioresistant cells became even more pronounced (Figure 3B). We conducted a preliminary experiment using A549/X cells infected with the COX8-EGFP-mCherry plasmid to determine the optimal MOI for achieving the desired transfection efficiency and found that the best infection effect was achieved when MOI =80 (Figure 3C). Confocal microscopy was performed after transfecting the cells with the plasmids and treating the cells with or without irradiation. The results showed that, compared with that in parent cells, the number of red dots exhibited mitophagy in radioresistant cells increased. Furthermore, after radiation exposure, the increase of the number of red dots indicating mitophagy in radioresistant cells was even more pronounced (Figure 3D). We also discovered a positive correlation between the FOXO3a gene expression level and the radioresistance of NSCLC. Compared with those in the parental cell lines, the basal levels of FOXO3a gene mRNA (message RNA) and protein were higher in NSCLC radioresistant cell lines. After irradiation, the reactive increase in FOXO3a gene mRNA and protein expression was more prominently in radioresistant cells (Figure 3E,3F).
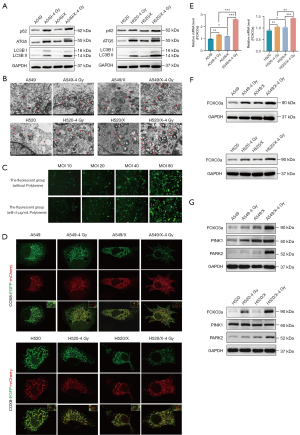
We further examined the major regulatory mechanism of mitophagy, the PINK1/Parkin pathway. It was found that the basal levels of mitochondrial autophagy-related genes and the PINK1/Parkin pathway were higher in NSCLC radioresistant cell lines than in the parental cell lines. After radiotherapy, the expression levels of mitochondrial autophagy-related genes and PINK1/Parkin pathway proteins were significantly higher in radioresistant cell lines (Figure 3G), indicating a close correlation between mitophagy and radioresistance in NSCLC.
Furthermore, we found that high expression of the PINK1 gene in patients with lung adenocarcinoma or LUSC in TCGA database was associated with shorter OS, DSS, and PFI, with differences being statistically significant (Figure S1B). In addition, LUSC patients with high PRKN gene expression also demonstrated a shorter OS (Figure S1C). The high expression of the FOXO3a-PINK1/Parkin pathway may be one of the adverse factors restricting the survival and prognosis of patients with NSCLC, and based on the data (Figures 2,3), we speculate that the FOXO3a-PINK1/Parkin pathway may play an important role in regulating radiotherapy resistance in NSCLC.
Association of FOXO3a gene expression and the radioresistance of NSCLC cells
We knocked down the FOXO3a gene in the A549/X and H520/X radioresistant cell lines using siRNA and then employed various experimental methods to detect changes in the radioresistance of these cells, with the aim of investigating the relationship between the expression level of the FOXO3a gene and the radioresistance of NSCLC radioresistant cell lines. We examined the transfection concentration and efficiency of three siRNA sequences (sequence#1, sequence#2, and sequence#3) targeting the FOXO3a gene in A549/X cells (Figure 4A). We found that all three siRNAs exhibited efficacy of knocking down FOXO3a expression, and the transfection efficiency was highest at 100 nM of siRNA concentration. We further validated the transfection efficiency of these three FOXO3a siRNAs for knocking down the FOXO3a gene in both A549/X and H520/X cells (Figure 4B). The results showed that siRNA#1 and siRNA#3 had the best interference effects on both cell lines and thus, could be used for subsequent experiments.
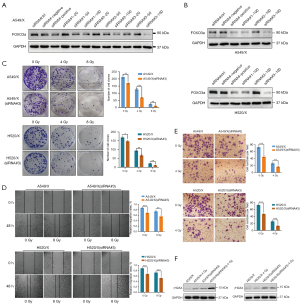
We used various experimental methods to detect changes in the radioresistance of A549/X and H520/X radioresistant cells. After knockdown of the FOXO3a gene via siRNA, the clone formation experiment showed that the clone formation ability of the radioresistant cells was significantly reduced, and their radiation tolerance and cell proliferation ability were weakened (Figure 4C); the wound-healing assay indicated a decreased migratory capacity of the cells (Figure 4D), and the Transwell assay revealed a poor invasive ability of the radioresistant cells (Figure 4E). Additionally, the ability of the radioresistant cells to resist X-ray-induced DNA damage was also significantly reduced (Figure 4F).
The expression level of the FOXO3a gene correlates to PINK1/Parkin pathway and the mitochondrial autophagy in vitro
We further used siRNA to knockdown the FOXO3a gene in A549/X and H520/X radioresistant cells and then employed various experimental methods to examine the changes in the expression levels of mitochondrial autophagy and the PINK1/Parkin pathway, with the aim to ascertain the relationship between the expression level of the FOXO3a gene and the mitochondrial autophagy PINK1/Parkin pathway in NSCLC radioresistant cell lines. Using Western blot analysis (Figure 5A), TEM (Figure 5B), combining with transfecting cells with COX8-EGFP-mCherry fluorescent plasmids (Figure 5C), we investigated the changes in mitochondrial autophagy reactions in cells before and after irradiation. We found that after the downregulation of the FOXO3a gene in NSCLC radioresistant cells, the basal level of mitochondrial autophagy within the cells was lower when compared to its original state, and the ability of mitochondrial autophagy to be activated by X-ray radiation was diminished compared to that in the original resistant cells. Additionally, when the FOXO3a gene in NSCLC radioresistant cell lines was knocked down by siRNA, the basal level of the PINK1/Parkin pathway in mitochondrial autophagy was lower than the original state, and the reactive upregulation of this pathway in response to radiotherapy was also attenuated compared to that of the original resistant cells (Figure 5D).
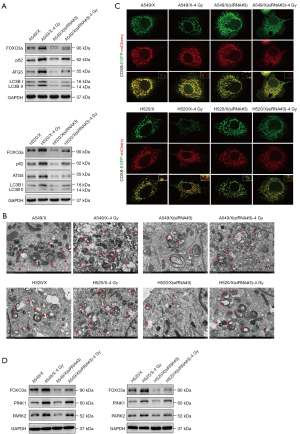
The regulatory role of FOXO3a gene in NSCLC radioresistance through the mitochondrial autophagy PINK1/Parkin pathway in vivo
The preliminary data of our study confirmed that the regulation of FOXO3a on the radioresistance of NSCLC may be achieved through modulating the mitochondrial autophagy PINK1/Parkin pathway at the cellular level. We aimed to confirm this by using nude mouse xenograft tumor models. Consequently, three different sequences of FOXO3a-sh plasmids were selected, extracted, and transfected into HEK293T cells to obtain virus solutions, which were then used to infect A549/X and H520/X cells to construct stable NSCLC radioresistant cell lines with knocked-down FOXO3a. Significant and stable fluorescence was observed in the radiotherapy-resistant cell lines A549/X and H520/X that were infected with sh-NC (control shRNA) plasmids and the three plasmids of FOXO3a-sh1/2/3 (Figure 6A). Western blot analysis showed that FOXO3a-sh3 exhibited the highest knockdown efficiency for the FOXO3a gene, and the expression of mitochondrial autophagy PINK1/Parkin pathway proteins was significantly reduced after FOXO3a gene knockdown (Figure 6B).
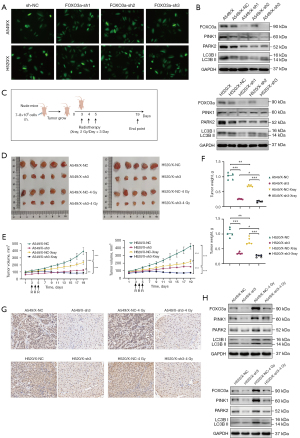
We established nude mouse xenograft tumor models using the A549/X-NC, A549/X-sh-FOXO3a, H520/X-NC, and H520/X-sh-FOXO3a cell lines. After random grouping, the nude mice in the treatment group received local X-ray radiotherapy at 2 Gy/day for 3 consecutive days on the subcutaneous tumors, while the control group did not receive X-ray radiotherapy (Figure 6C). The growth or regression of the tumors was recorded during the process, and the experiment was terminated 14 days after radiotherapy, with the tumors being excised, photographed (Figure 6D), and weighed. In the control group, the volume and weight of A549/X-sh3 xenograft tumors were lower when compared to those of the A549/X-NC xenograft tumors, and the same was observed in the H520/X-sh3 and H520/X-NC xenograft tumors. Furthermore, in the radiotherapy group, the reduction in tumor volume and weight was more significant in the FOXO3a-sh3 cell xenograft tumors after radiotherapy (Figure 6E,6F). These results suggest that knocking down the FOXO3a gene and PINK1/Parkin pathway significantly weakens the tumorigenic ability of radioresistant NSCLC cells and significantly reduces their resistance to radiation. We also confirmed the positive regulatory effect of the FOXO3-PINK1/Parkin pathway on radioresistance to NSCLC in xenograft tumors through immunohistochemistry and Western blot assays (Figure 6G,6H).
Discussion
Radiotherapy is the cornerstone in the management of various pathological subtypes and stages of lung cancer and one of the most effective local treatment modalities in NSCLC. Nevertheless, the emergence of radioresistance—a phenomenon observed irrespective of radiotherapy techniques or dose fractionation regimens—poses a substantial clinical challenge that frequently compromises definitive tumor control. The molecular basis of NSCLC radioresistance involves intricate interplay among multiple genetic factors and complex regulatory mechanisms (26). Mitophagy, a selective form of autophagy, plays a critical role in mitochondrial quality control by selectively eliminating damaged mitochondria while maintaining proper mitochondrial homeostasis (27). Emerging evidence suggests that tumor cells exploit mitophagy to enhance cellular adaptation to various stress conditions, including nutrient deprivation, hypoxia, and radiation exposure. This adaptive response confers cytoprotective effects that promote cancer cell survival and ultimately contribute to tumor progression (28). Elucidating the precise mechanistic contributions of mitophagy in the development of radioresistance may provide crucial insights for the development of novel targeted therapeutic strategies against NSCLC. Such understanding could facilitate the identification of innovative molecular targets to overcome treatment resistance in clinical oncology.
In this work, we first induced and screened for stable A549 and H520 cells with radiation resistance and verified their radioresistance capabilities from multiple perspectives through various experiments, providing a basic experimental model foundation for subsequent exploration of the molecular mechanisms of radioresistance, which may help obtain a deeper understanding of the biological mechanisms of radioresistance. In addition, the cell biological and molecular biological experimental validation methods used in this study to examine the properties of radioresistance in NSCLC cells will also be applied in future research. Then we performed high-throughput transcriptomic sequencing and comparative analysis of the NSCLC baseline cell lines with and without radiotherapy and of the radioresistant cell lines. We found that the genes related to mitophagy in the A549/X and H520/X radioresistant cell lines were significantly enriched compared to the baseline cell lines after radiation exposure; among the enriched genes, the FOXO3a gene demonstrated the most significant enhancement. This suggests that FOXO3a may be closely related to mitophagy and to the radioresistance of NSCLC radioresistant cell lines.
Among the complex molecular mechanisms regulating mitophagy, our results suggest that the PINK1/Parkin pathway may be considered the primary regulatory route. PINK1 triggers the binding of ubiquitin ligase to phosphorylated ubiquitin by phosphorylating Parkin and ubiquitin, thus promoting the recruitment and activation of Parkin, forming a feedback loop. Specific adaptor proteins (such as p62, OPTN, NDP52, and TAXBP1) interact with activated PINK1/Parkin on the outer mitochondrial membrane (OMM) and bind to ubiquitin and LC3 for autophagic degradation (29). Mitochondria increase ionizing radiation-induced DNA damage by downregulating or overexpressing the key mitochondrial phagocytic proteins Parkin and BNIP3. X-rays can enhance the basal level of mitochondrial necrosis; potentially induce cell cycle arrest in the G2/M phase; significantly increase the accumulation of γ-H2AX, 53BP1, and PARP1 lesions in the nucleus; increase DNA damage; and promote tumor cell death (30). A study has found that PINK1/Parkin promotes tumor growth and development as well as resistance to radiotherapy and chemotherapy. PINK1/Parkin-mediated overactivation of mitochondrial phagocytosis enhances DNA damage repair, thereby promoting tumor cell resistance to radiation (31).
Moreover, we found that patients with NSCLC and a high expression of FOXO3a-PINK1/Parkin pathway gene showed shorter OS, DSS, and PFI. The high expression of FOXO3a-PINK1/Parkin pathway gene may be one of the unfavorable factors restricting the survival and prognosis of patients with NSCLC, and it is also an important regulator of radiotherapy resistance in NSCLC cells.
Cellular mutations are often accompanied by mitochondrial damage and activation of the mitophagy system, and the direct mutual activation of PINK1 and Parkin may promote tumorigenesis. Programmed necrosis of endothelial cells and tumor cells under the action of PINK1/Parkin can promote tumor cell metastasis (32). The high expression of E3 ubiquitin ligase ARIH1 in cancer cells can trigger mitophagy in a PINK1-dependent manner; this counteracts chemotherapy-induced cell death in breast cancer and lung adenocarcinoma, ultimately leading to tumor cell resistance (33). Through analysis and validation of high-throughput transcriptomic sequencing, we found that the basal levels of mitophagy-related genes and the PINK1/Parkin pathway in NSCLC radioresistant cells were higher than those in the baseline cells. Following the administration of radiotherapy, the expression levels of mitophagy-related genes and PINK1/Parkin pathway proteins in radioresistant cells were more significantly enhanced, indicating that the PINK1/Parkin pathway of mitophagy is closely related to NSCLC radioresistance. Dysregulation of mitophagy may lead to the accumulation of damaged mitochondria, which plays an important role in the development and progression of tumors. Therefore, the complex role and regulatory mechanism of mitochondrial phagocytosis in different tumor environments represent a promising target to explore in terms of antitumor therapy (34). In our study, we also observed a consistent correlated reactive upregulation of both PINK1/Parkin pathway regulating the mitochondrial autophagy and the FOXO3a gene: the basal levels of the FOXO3a protein and PINK1/Parkin pathway proteins in NSCLC radioresistant cells were higher than those in baseline cells; after radiotherapy intervention, the expression levels of both the FOXO3a protein and PINK1/Parkin pathway proteins were more significantly increased, showing a clear consistency in their reactive upregulation enhancement. The FOXO3a gene is widely distributed in human tissues and as the most prominent member of the mammalian FOXO family, it is closely related to the development of various diseases and participates in multiple physiological and pathological processes of tumor cells (16). There is no conclusive data regarding the regulatory role of the FOXO3a gene in the development of lung cancer (35). On the one hand, it has been reported that the FOXO3a gene may act as a tumor suppressor in lung cancer: FOXO3a gene inactivation often occurs in carcinogen-induced lung adenocarcinoma (36), the overexpression of FOXO3 can inhibit the promotion of cisplatin resistance in NSCLC mediated by lactate (37), and miR-155 inhibits FOXO3a and enhances the resistance of lung cancer cells to gefitinib, while overexpression of FOXO3a in gefitinib-resistant lung cancer cells ameliorates this effect (38). On the other hand, FOXO3a also promotes the development of NSCLC and other tumors. Overexpression of FOXO3a is associated with some unfavorable clinicopathological features of multiple tumors (39-41), such as advanced tumor-node-metastasis (TNM) staging, histological grade, and vascular invasion, and serves as an important indicator for predicting clinical prognosis. In addition, the expression level of FOXO3a is positively correlated with the progression of glioblastoma (42). It has also been reported that FOXO3a can enhance the resistance of glioma cells to temozolomide by promoting the accumulation of β-catenin in the nucleus (20). FOXO3a may also play a role in tumor radiotherapy and is associated with tumor cell radiosensitivity and resistance. Overexpression of FOXO3a reduces the level of glycolysis and alleviates the radioresistance induced by enhancing miR-223-3p expression to a certain extent (43). Chen et al. found that nuclear accumulation of FOXO3a in esophageal cancer cells is associated with increased radiosensitivity, indicating its great potential as a biomarker for predicting the response of patients with esophageal cancer to radiation (44). However, the potential of FOXO3a as a biomarker for radiotherapy resistance in patients with cancer has not been extensively reported, and further experiments and clinical studies are needed to confirm its potential role in this scenario.
After knocking down the FOXO3a gene in A549/X and H520/X radioresistant cell lines using siRNA, we observed a significant reduction in the clonogenic potential, cell migration ability, cell invasion capacity, and resistance to radiation-induced DNA damage of the resistant cells. This indicates a close association between the expression level of the FOXO3a gene and the radioresistance of NSCLC radioresistant cells. Furthermore, following the knockdown of the FOXO3a gene in NSCLC radioresistant cells, the baseline level of mitophagy and the expression of the PINK1/Parkin pathway proteins were significantly lower compared to the original resistant cells. The ability of X-rays to activate the mitophagy PINK1/Parkin pathway was greatly reduced, and the reactive upregulation of related proteins in this pathway was also weaker than that in the original resistant cells. Therefore, it can be concluded that there is a close, positive correlation between the expression level of the FOXO3a gene in NSCLC radioresistant cells and the degree of mitophagy and expression level of the PINK1/Parkin pathway. The regulation of NSCLC radioresistance by FOXO3a may be achieved through modulating the mitophagy PINK1/Parkin pathway.
We further validated the above-mentioned conclusions through in vivo experiments using animal models. By constructing xenograft tumor models in nude mice and administrating radiotherapy treatment to control cells and NSCLC radioresistant cells with stable knockdown of the FOXO3a gene and reduced expression of PINK1/Parkin pathway proteins, we found that the expression level of the FOXO3a gene was positively correlated with the proliferation and tumorigenic ability of NSCLC radioresistant cells. Moreover, tumor regression following radiotherapy was more pronounced in the xenograft tumors derived from cells with the FOXO3a gene knockdown. Therefore, we confirmed both in vitro and in vivo that the FOXO3a gene regulates the radioresistance of NSCLC through the mitochondrial autophagy PINK1/Parkin pathway.
Our study provides preliminary evidence that activation of the FOXO3a-PINK1/Parkin pathway enhances radioresistance in non-small cell lung cancer (NSCLC), a finding that may offer novel therapeutic opportunities in clinical radiotherapy practice, and the pathway demonstrates dual clinical potential. Firstly, it may serve as a predictive biomarker for radioresistance and quantitative assessment of pathway component expression levels could help predict radiotherapy response in NSCLC patients. Furthermore, the pathway represents a promising therapeutic target and development of safe and effective FOXO3a inhibitors could potentially radiosensitize NSCLC cells and improve treatment outcomes. The FOXO3a-PINK1/Parkin pathway represents a novel therapeutic paradigm for overcoming tumor radioresistance; however, its clinical translation necessitates resolution of critical challenges including target specificity and biomarker standardization. Further translational development will require extensive preclinical validation and rigorously controlled clinical trials.
Conclusions
To our knowledge, this is the first study to establish the crucial role of FOXO3a in regulating the radioresistance of NSCLC cells through mitochondrial autophagy mediated by the PINK1/Parkin pathway. The design and screening of specific inhibitors that precisely target the FOXO3a gene, as well as the translation of our experimental results into clinical applications, thereby developing novel drugs that may benefit patients with NSCLC who develop radioresistance after radiotherapy, represent important directions for our future research efforts.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE and MDAR reporting checklists. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-181/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-181/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-181/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-181/coif). C.Z. has grant support from AstraZeneca for conducting a clinical trial on NSCLC, is a cofounder of ZCure LLC as an inventor of US provisional patent that is currently PCT pending, and serves as a board member of DSMB at University of Nebraska Medical Center. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Animal experiments were performed under a project license (No. K24-206Y) approved by Institutional Animal Care and Use Committee of Shanghai Pulmonary Hospital, in compliance with the Shanghai Pulmonary Hospital guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bradley JD, Hu C, Komaki RR, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:706-14. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Barker HE, Paget JT, Khan AA, et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409-25. [Crossref] [PubMed]
- Wu L, Zhang Z, Bai M, et al. Radiation combined with immune checkpoint inhibitors for unresectable locally advanced non-small cell lung cancer: synergistic mechanisms, current state, challenges, and orientations. Cell Commun Signal 2023;21:119. [Crossref] [PubMed]
- Gong X, Li X, Jiang T, et al. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1085-97. [Crossref] [PubMed]
- Yang P, Feng X, Li J, et al. Ionizing radiation downregulates estradiol synthesis via endoplasmic reticulum stress and inhibits the proliferation of estrogen receptor-positive breast cancer cells. Cell Death Dis 2021;12:1029. [Crossref] [PubMed]
- Yang P, Luo X, Li J, et al. Ionizing Radiation Upregulates Glutamine Metabolism and Induces Cell Death via Accumulation of Reactive Oxygen Species. Oxid Med Cell Longev 2021;2021:5826932. [Crossref] [PubMed]
- Naik PP, Birbrair A, Bhutia SK. Mitophagy-driven metabolic switch reprograms stem cell fate. Cell Mol Life Sci 2019;76:27-43. [Crossref] [PubMed]
- Chourasia AH, Boland ML, Macleod KF. Mitophagy and cancer. Cancer Metab 2015;3:4. [Crossref] [PubMed]
- Drake LE, Springer MZ, Poole LP, et al. Expanding perspectives on the significance of mitophagy in cancer. Semin Cancer Biol 2017;47:110-24. [Crossref] [PubMed]
- Chang JY, Yi HS, Kim HW, et al. Dysregulation of mitophagy in carcinogenesis and tumor progression. Biochim Biophys Acta Bioenerg 2017;1858:633-40. [Crossref] [PubMed]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011;12:9-14. [Crossref] [PubMed]
- Liu L, Zuo Z, Lu S, et al. Silencing of PINK1 represses cell growth, migration and induces apoptosis of lung cancer cells. Biomed Pharmacother 2018;106:333-41. [Crossref] [PubMed]
- Yamashita K, Miyata H, Makino T, et al. High Expression of the Mitophagy-Related Protein Pink1 is Associated with a Poor Response to Chemotherapy and a Poor Prognosis for Patients Treated with Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2017;24:4025-32. [Crossref] [PubMed]
- Zhang Y, Pang C, Zhang C, et al. HILPDA-mediated lipidomic remodelling promotes radiotherapy resistance in nasopharyngeal carcinoma by accelerating mitophagy. Cell Mol Life Sci 2023;80:242. [Crossref] [PubMed]
- Liu Y, Ao X, Ding W, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer 2018;17:104. [Crossref] [PubMed]
- Fan X, Cui L, Zeng Y, et al. 14-3-3 Proteins Are on the Crossroads of Cancer, Aging, and Age-Related Neurodegenerative Disease. Int J Mol Sci 2019;20:3518. [Crossref] [PubMed]
- Habrowska-Górczyńska DE, Kozieł MJ, Kowalska K, et al. FOXO3a and Its Regulators in Prostate Cancer. Int J Mol Sci 2021;22:12530. [Crossref] [PubMed]
- Zhao F, Lam EW. Role of the forkhead transcription factor FOXO-FOXM1 axis in cancer and drug resistance. Front Med 2012;6:376-80. [Crossref] [PubMed]
- Xu K, Zhang Z, Pei H, et al. FoxO3a induces temozolomide resistance in glioblastoma cells via the regulation of β-catenin nuclear accumulation. Oncol Rep 2017;37:2391-7. [Crossref] [PubMed]
- Chen G, Yu L, Dong H, et al. MiR-182 enhances radioresistance in non-small cell lung cancer cells by regulating FOXO3. Clin Exp Pharmacol Physiol 2019;46:137-43. [Crossref] [PubMed]
- Zhou Y, Chen E, Tang Y, et al. miR-223 overexpression inhibits doxorubicin-induced autophagy by targeting FOXO3a and reverses chemoresistance in hepatocellular carcinoma cells. Cell Death Dis 2019;10:843. [Crossref] [PubMed]
- He C, Lu S, Wang XZ, et al. FOXO3a protects glioma cells against temozolomide-induced DNA double strand breaks via promotion of BNIP3-mediated mitophagy. Acta Pharmacol Sin 2021;42:1324-37. [Crossref] [PubMed]
- Yao J, Wang J, Xu Y, et al. CDK9 inhibition blocks the initiation of PINK1-PRKN-mediated mitophagy by regulating the SIRT1-FOXO3-BNIP3 axis and enhances the therapeutic effects involving mitochondrial dysfunction in hepatocellular carcinoma. Autophagy 2022;18:1879-97. [Crossref] [PubMed]
- Wang Y, Meng L, Meng S, et al. Flotillin-1 enhances radioresistance through reducing radiation-induced DNA damage and promoting immune escape via STING signaling pathway in non-small cell lung cancer. Cancer Biol Ther 2023;24:2203332. [Crossref] [PubMed]
- Suwa T, Kobayashi M, Nam JM, et al. Tumor microenvironment and radioresistance. Exp Mol Med 2021;53:1029-35. [Crossref] [PubMed]
- Pickles S, Vigié P, Youle RJ. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr Biol 2018;28:R170-85. [Crossref] [PubMed]
- Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxic mitochondria: accomplices in resistance. Bull Cancer 2011;98:40-6. [Crossref] [PubMed]
- Poole LP, Macleod KF. Mitophagy in tumorigenesis and metastasis. Cell Mol Life Sci 2021;78:3817-51. [Crossref] [PubMed]
- Ren Y, Yang P, Li C, et al. Ionizing radiation triggers mitophagy to enhance DNA damage in cancer cells. Cell Death Discov 2023;9:267. [Crossref] [PubMed]
- Wei Y, Xiao G, Xu H, et al. Radiation resistance of cancer cells caused by mitochondrial dysfunction depends on SIRT3-mediated mitophagy. FEBS J 2023;290:3629-45. [Crossref] [PubMed]
- Bernardini JP, Lazarou M, Dewson G. Parkin and mitophagy in cancer. Oncogene 2017;36:1315-27. [Crossref] [PubMed]
- Villa E, Proïcs E, Rubio-Patiño C, et al. Parkin-Independent Mitophagy Controls Chemotherapeutic Response in Cancer Cells. Cell Rep 2017;20:2846-59. [Crossref] [PubMed]
- Dong Y, Zhang X. Targeting cellular mitophagy as a strategy for human cancers. Front Cell Dev Biol 2024;12:1431968. [Crossref] [PubMed]
- Ebrahimnezhad M, Valizadeh A, Majidinia M, et al. Unveiling the potential of FOXO3 in lung cancer: From molecular insights to therapeutic prospects. Biomed Pharmacother 2024;176:116833. [Crossref] [PubMed]
- Blake DC Jr, Mikse OR, Freeman WM, et al. FOXO3a elicits a pro-apoptotic transcription program and cellular response to human lung carcinogen nicotine-derived nitrosaminoketone (NNK). Lung Cancer 2010;67:37-47.
- Bo W, Yu N, Wang X, et al. Lactate promoted cisplatin resistance in NSCLC by modulating the m6A modification-mediated FOXO3/MAGI1-IT1/miR-664b-3p/IL-6R axis. Neoplasia 2024;48:100960. [Crossref] [PubMed]
- Correction for Chiu et al. NF-κB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc Natl Acad Sci U S A 2017;114:E654-5. [Crossref] [PubMed]
- Rehman A, Kim Y, Kim H, et al. FOXO3a expression is associated with lymph node metastasis and poor disease-free survival in triple-negative breast cancer. J Clin Pathol 2018;71:806-13. [Crossref] [PubMed]
- Ahn H, Kim H, Abdul R, et al. Overexpression of Forkhead Box O3a and Its Association With Aggressive Phenotypes and Poor Prognosis in Human Hepatocellular Carcinoma. Am J Clin Pathol 2018;149:117-27. [Crossref] [PubMed]
- Zhang G, Shi W, Jia E, et al. FOXO3A Expression in Upper Tract Urothelial Carcinoma. Front Oncol 2021;11:603681. [Crossref] [PubMed]
- Qian Z, Ren L, Wu D, et al. Overexpression of FoxO3a is associated with glioblastoma progression and predicts poor patient prognosis. Int J Cancer 2017;140:2792-804. [Crossref] [PubMed]
- Zhou K, Wei Y, Li X, et al. MiR-223-3p targets FOXO3a to inhibit radiosensitivity in prostate cancer by activating glycolysis. Life Sci 2021;282:119798. [Crossref] [PubMed]
- Chen MF, Fang FM, Lu CH, et al. Significance of nuclear accumulation of Foxo3a in esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2008;71:1220-9. [Crossref] [PubMed]


