
Metabolic tumor volume on 18F-FDG uptake as a negative predictor after ipilimumab plus nivolumab in advanced non-small cell lung cancer
Highlight box
Key findings
• High metabolic tumor volume (MTV) and total lesion glycolysis (TLG) on 2-deoxy-2-[fluorine-18]-fluoro-d-glucose (18F-FDG) positron emission tomography (PET) were significantly associated with poor performance status (PS), no response, high neutrophil-to-leukocyte ratio (NLR), low albumin levels, and high C-reactive protein (CRP) level.
• MTV on 18F-FDG uptake was identified as a negative predictor in non-small cell lung cancer (NSCLC) patients receiving nivolumab plus ipilimumab (Nivo-Ipi).
• MTV as predictor was helpful on the population of pulmonary adenocarcinoma or programmed death ligand-1 (PD-L1) <1%.
• A high MTV with poor outcome was closely associated with a poor PS, lymph node metastases, bone metastases, high NLR, low albumin levels, and high CRP level.
What is known and what is new?
• Expression of PD-L1 is not a significant predictor on the therapeutic outcome of Nivo-Ipi treatment. But, Nivo-Ipi improved the long-term survival in patients with PD-L1 <1%.
• Tumor metabolic volume may be associated with resistance to Nivo-Ipi therapy.
What is the implication, and what should change now?
• MTV on 18F-FDG-PET at baseline Nivo-Ipi could successfully predict the prognosis of this treatment. In particular, we identified the usefulness of 18F-FDG-PET as predictive marker for the group with PD-L1 <1%.
Introduction
Immune checkpoint inhibitors (ICIs) are widely administered as best treatment for patients with recurrent or metastatic non-small cell lung cancer (NSCLC). The regimens including programmed death-1 (PD-1) blockade is chosen as front-line treatment, based on programmed death ligand-1 (PD-L1) expression within tumor specimens. However, little is known about the predictive marker of an anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) antibody for all neoplasms. The results of the CheckMate 227 and CheckMate 9LA studies described a 5-year overall survival (OS) rate of approximately 20% in patients with NSCLC with PD-L1 <1% (1,2). Therefore, a combination of PD-1 and CTLA4 antibodies is a key regimen even in patients negative for PD-L1 expression. Further investigation is warranted to discover the optimal predictor for the effects of PD-1 plus CTLA4 antibody treatment (nivolumab plus ipilimumab: Nivo-Ipi).
We have previously reported that metabolic tumor activity on positron emission tomography (PET) at baseline treatment could predict the prognosis of PD-1 inhibitor in patients with NSCLC (3-6). Similarly, maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) are indicators for metabolic tumor activity in 2-deoxy-2-[fluorine-18]-fluoro-d-glucose (18F-FDG) uptake. Moreover, 18F-FDG accumulation based on MTV or TLG but not SUVmax may have prognostic significance in ICIs treatment (3-6). Thus, assessing tumor activity based on measures such as MTV or TLG may help inform therapeutic sensitivity in ICIs treatment.
MTV in pretreatment 18F-FDG PET is a significant prognostic marker and predictive of resistance to treatment in patients with advanced melanoma receiving Nivo-Ipi (7,8). However, aside from melanoma, the relationship between Nivo-Ipi efficacy and metabolic tumor activity in 18F-FDG PET remains unclear. In patients administered PD-1 blockade alone for the treatment of NSCLC, tumor PD-L1 expression is useful for predicting therapeutic efficacy (8). However, PD-L1 expression is not predictive of treatment efficacy for the combination of CTLA4 antibody and PD-1 blockade. Moreover, although PD-L1 expression is closely related to 18F-FDG uptake on PET (8), the association between CTLA4 expression and 18F-FDG accumulation in PET is not clearly understood. Therefore, investigating whether metabolic tumor activity based on 18F-FDG uptake can predict Nivo-Ipi treatment outcomes in patients with NSCLC may contribute to the development of predictive markers for CTLA4 antibody therapy.
In this context, the present retrospective study evaluated the prognostic relevance of 18F-FDG PET after Nivo-Ipi in patients with metastatic or advanced NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1084/rc).
Methods
Patients
This study included 130 patients with metastatic or advanced NSCLC administered Nivo-Ipi as a first-line treatment and underwent 18F-FDG PET prior to the initial treatment at our institution between December 2020 and December 2022. Some of these patients have been reported previously (9). Clinical information was collected based on the patients’ medical records. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Ethics Committee of the International Medical Center of Saitama Medical University (No. 20-125), which waived the requirement for written informed consent owing to the retrospective study design (10).
Treatment and assessment
Nivolumab (240 mg/day) and ipilimumab were intravenously administered once every 3 weeks and 1 mg/kg once every 6 weeks, respectively, as previously described (1,2). The chief physicians confirmed physical examinations, complete blood counts, and biochemical tests, and graded the side effects. Moreover, white blood cells, neutrophils, lymphocytes, and albumin were extracted within 1 week of the initial cycle of Nivo-Ipi. The inflammatory index was calculated as neutrophil-to-lymphocyte ratio (NLR) = neutrophil count/lymphocyte count. The optimal cut-off value for this blood marker was the median value, and values greater than this cut-off were defined as high (9). Toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0. Tumor response was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (11).
PET data analysis
PET imaging and data analysis: the patients were evaluated using a PET/computed tomography (CT) scanner (Siemens Healthineers Biograph Vision 600). The three-dimensional data acquisition was initiated 60 min after FDG injection. Eight bed positions were selected according to the imaging range. The attenuation-corrected transverse images were reconstructed using an ordered-subset expectation-maximization algorithm according to the point-spread function into 168 × 168 matrices with a 2.00-mm slice thickness.
For semi-quantitative analysis, SUV was assessed based on the injected dose, patient weight, and cross-calibration factor between PET and the dose calibrator. SUV was calculated as follows: SUV = radioactive concentration in the volume of interest (VOI) (MBq/g)/injected dose (MBq)/patient weight (g). CT for initial staging was done using an intravenous contrast medium. Board-certified radiologists interpreted the images. We utilized syngo.via (Siemens Healthineers Co. Ltd., Japan) on a Windows workstation to semi-automatically calculate the SUVmax and peak SUV (SUVpeak). For 18F-FDG PET, MTV and TLG, defined as the MTV multiplied by SUVmean for each lesion, were calculated using the SUV threshold obtained for the SUV of the liver VOI. Each threshold was defined as the average of 1.5 × SUV (SUVmean) plus 2 × SD of the liver SUV. These SUV thresholds were the optimum values with generating three-dimensional (3D) VOIs that completely enclosed the entire tumor mass, using CT images for reference. Regions of activity other than tumors, including the kidneys, urinary tract, myocardium, and gastrointestinal tract, were manually eliminated by a board-certified nuclear medicine physician according to the orientation provided. The liver VOI was automatically set using AI analysis software.
Statistical analysis
Statistical significance was evaluated at P<0.05. Fisher’s exact test was assessed to examine the association between categorical variables. The correlations between SUVmax, SUVpeak, MTV, TLG, and 18F-FDG uptake were analyzed by Pearson’s rank tests. Responders and non-responders based on RECIST were defined as having complete response (CR) or partial response (PR), and stable disease (SD) or progressive disease (PD), respectively. The optimal cut-off values for SUVmax and SUVpeak for 18F-FDG uptake were evaluated by receiver operating characteristic (ROC) curve analyses, and sensitivity and specificity were calculated to obtain the optimal cut-off value for differentiating responders from non-responders using ROC curves. Progression-free survival (PFS) was defined as the time from Nivo-Ipi treatment to disease progression or death. Overall survival (OS) was defined as the time from Nivo-Ipi treatment to death from any cause. The parameters for 18F-FDG uptake were compared using a cutoff for PFS of 6 months (<6 versus ≥6 months) and OS of 12 months (<12 versus ≥12 months). The Kaplan-Meier method was utilized to estimate survival as a function of time, and survival differences were analyzed based on the log-rank test. Univariate and multivariate analyses of different variables were performed using logistic regression. Immunohistochemical examination of tumor PD-L1 (22C3; DAKO, Carpinteria, CA, USA) expression levels were routinely assessed before Nivo-Ipi therapy and reported as tumor proportional scores. These scores were then classified into three groups: <1%, 1–49%, and ≥50%. All statistical analyses were performed using GraphPad Prism (v.7.0e; GraphPad Software, San Diego, CA, USA) and JMP Pro 16.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics and PET findings
Table 1 presents the patient demographics based on 18F-FDG uptake. The median 18F-FDG uptake values at baseline were 11.6 (range, 1.8–37.6) for SUVmax, 9.6 (range, 1.5–32.1) for SUVpeak, 76.2 (range, 1.4–994.6) cm3 for MTV, and 356.2 (range, 5.9–10,255.8) g*cm3/mL for TLG. The optimal cut-off values for SUVmax, SUVpeak, MTV, and TLG on 18F-FDG uptake, as determined by ROC curve analyses, were 9.5 (sensitivity: 72.3%; specificity: 37.4%), 8.2 (sensitivity: 72.3%; specificity: 45.8%), 143.0 (sensitivity: 89.3%; specificity: 32.6%), and 695.0 (sensitivity: 82.9%; specificity: 37.4%), respectively. The area under the curve values for ROC were 0.54 for SUVmax and 0.57 for SUVpeak, 0.55 for MTV, and 0.54 for TLG. The median duration from 18F-FDG PET scanning to initiation of Nivo-Ipi treatment was 28 days (range, 1–91 days). The median patient age was 71 years (range, 43–81 years). Men and women comprised 107 and 23 patients, respectively. Overall, 47, 65, 14, and 4 patients had a performance status (PS) of 0, 1, 2, and 3, respectively. The histological examination revealed that 70, 42, and 18 patients had (AC), squamous cell carcinoma (SQC), and other types, respectively. The PD-L1 expression levels were <1%, 1–49%, and ≥50% in 66, 55, and 9 patients, respectively. The patient’s demographics according to 18F-FDG uptake are presented in Table 1. High MTV and TLG were closely linked to poor PS, bone metastases, non-responder, low incidence of any immune-related adverse events (irAEs), high NLR, high leukocyte counts, high neutrophil counts, low albumin levels, and high CRP level. High SUVmax and SUVpeak were significantly associated with a history of heavy smoking and non-AC. High MTV was significantly associated with high PD-L1 expression and a history of heavy smoking.
Table 1
| Different variables | All patients (n=130) | SUVmax | SUVpeak | MTV | TLG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High (n=87) | Low (n=43) | P value | High (n=79) |
Low (n=51) | P value | High (n=33) | Low (n=97) | P value | High (n=40) | Low (n=90) | P value | |||||
| Age (<75/≥75 years) | 89/41 | 60/27 | 29/14 | >0.99 | 54/25 | 35/16 | >0.99 | 25/8 | 64/33 | 0.38 | 30/10 | 59/31 | 0.31 | |||
| Sex (male/female) | 107/23 | 73/14 | 34/9 | 0.62 | 67/12 | 40/11 | 0.35 | 30/3 | 77/20 | 0.18 | 36/4 | 71/19 | 0.14 | |||
| ECOG PS (0–1/2–3) | 113/17 | 73/14 | 40/3 | 0.17 | 66/13 | 47/4 | 0.18 | 22/11 | 91/6 | 0.001* | 27/13 | 86/4 | 0.001* | |||
| Smoking (BI) (820</≥820) | 74/56 | 56/31 | 18/25 | 0.02* | 28/51 | 46/15 | 0.001* | 19/14 | 55/42 | >0.99 | 16/24 | 58/32 | 0.01* | |||
| Histology (AC/non-AC) | 70/60 | 35/52 | 8/35 | 0.01* | 31/48 | 39/12 | 0.001* | 18/15 | 52/45 | >0.99 | 21/11 | 49/41 | 0.30 | |||
| Lymph meta (yes/no) | 99/31 | 68/19 | 31/12 | 0.51 | 62/17 | 37/14 | 0.52 | 27/6 | 72/25 | 0.48 | 34/6 | 65/25 | 0.12 | |||
| Brain meta (yes/no) | 22/108 | 16/71 | 6/37 | 0.62 | 14/65 | 8/43 | 0.81 | 6/27 | 16/81 | 0.79 | 5/35 | 17/73 | 0.45 | |||
| Bone meta (yes/no) | 46/84 | 34/53 | 12/31 | 0.24 | 30/49 | 16/35 | 0.45 | 21/12 | 25/72 | 0.001* | 23/17 | 23/67 | 0.001* | |||
| Response (PR/non-PR) | 47/83 | 34/53 | 13/30 | 0.34 | 34/45 | 13/38 | 0.06 | 6/27 | 41/56 | 0.01* | 9/31 | 38/52 | 0.047* | |||
| PD-L1 (%) (≥1/<1) | 51/79 | 36/51 | 15/28 | 0.56 | 33/46 | 18/33 | 0.58 | 14/19 | 37/60 | 0.68 | 19/11 | 32/58 | 0.01* | |||
| Any irAEs (yes/no) | 88/42 | 58/29 | 30/13 | 0.84 | 52/27 | 36/15 | 0.70 | 14/19 | 74/23 | <0.001* | 20/20 | 68/22 | 0.007* | |||
| G3/4 irAEs (yes/no) | 48/82 | 30/57 | 18/25 | 0.44 | 27/52 | 21/30 | 0.45 | 9/24 | 39/58 | 0.21 | 12/18 | 36/54 | >0.99 | |||
| NLR (high/low) (≥2.8/<2.8) | 98/32 | 65/22 | 33/10 | >0.99 | 59/20 | 39/12 | >0.99 | 30/3 | 68/29 | 0.01* | 35/5 | 63/27 | 0.045* | |||
| Leukocyte (high/low) | 65/65 | 46/41 | 19/24 | 0.45 | 43/36 | 22/29 | 0.28 | 22/11 | 43/54 | 0.042* | 26/14 | 39/51 | 0.03* | |||
| Neutrophil (high/low) | 65/65 | 46/41 | 19/24 | 0.45 | 43/36 | 22/29 | 0.28 | 22/11 | 43/54 | 0.042* | 26/14 | 39/51 | 0.03* | |||
| Lymphocyte (high/low) | 64/66 | 45/42 | 19/24 | 0.45 | 41/38 | 23/28 | 0.47 | 14/19 | 50/47 | 0.42 | 18/22 | 46/44 | 0.57 | |||
| Albumin (high/low) | 64/66 | 39/48 | 25/18 | 0.19 | 33/46 | 31/20 | 0.047* | 4/29 | 60/37 | 0.001* | 8/32 | 56/34 | 0.001* | |||
| CRP (high/low) | 65/65 | 45/42 | 20/23 | 0.70 | 43/36 | 22/29 | 0.28 | 27/6 | 38/59 | 0.001* | 32/8 | 33/57 | 0.001* | |||
*, statistically significance. AC, adenocarcinoma; BI, brinkman index; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; irAEs, immune-related adverse events; lymph, lymph node; meta, metastasis; MTV, metabolic tumor volume; NLR, neutrophil to lymphocyte ratio; PS, performance status; PD-L1, programmed death ligand-1; PD-1, programmed death-1; PR, partial response; Prior RT, radiation before initial treatment; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis.
Among 122 patients with an evaluable lesion for response, the objective response rate (ORR) was 38.5% [47 PR, 39 SD, 36 PD, and 8 not evaluable (NE)], whereas, the disease control rate was 70.5%.
Relationship between 18F-FDG uptake and different variables
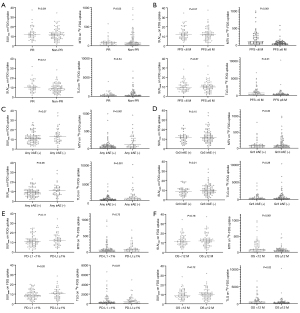
The SUVmax, SUVpeak, MTV, and TLG values on 18F-FDG uptake were compared according to the following variables; PR versus non-PR, PFS <6 months versus PFS ≥6 months, any irAE (+) versus any irAE (−), grade 3 irAEs (+) versus grade 3 irAEs (−), PD-L1 <1% versus PD-L1 ≥1%, OS <12 months versus OS ≥12 months (Figure 1). MTV was significantly lower in patients with PR, PFS ≥6 months, having any irAE, and OS ≥12 months compared with those without these factors. TLG was significantly lower in patients with PFS ≥6 months, having any irAE, and OS ≥12 months compared with those without these factors. SUVmax and SUVpeak values were not closely correlated with PR, PFS, OS, or irAEs. 18F-FDG uptake did not differ significantly between patients with PD-L1 <1% and PD-L1 ≥1%.
Survival analysis based on 18F-FDG uptake
Median PFS and OS were 202 and 548 days, respectively. One hundred patients experienced tumor recurrence, and 71 died due to disease progression. The Kaplan-Meier curves for PFS and OS according to the 18F-FDG uptake are depicted in Figure 2.
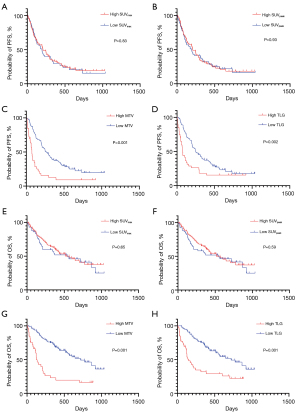
Univariate analysis of all patients identified PS, bone metastases, NLR, MTV, and TLG as significant predictors of PFS and OS (Table 2). The univariate log-rank test enabled screening for variables with P<0.05 for subsequent multivariate analysis. As TLG was calculated based on the MTV and was identified as a strong confounding factor, MTV was used in the multivariate analysis. The results of the multivariate analysis identified NLR and MTV as independent predictors of PFS and OS (Table 2).
Table 2
| Different variables | Progression-free survival | Overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| MST (days) | P value | HR | 95% CI | P value | MST (days) | P value | HR | 95% CI | P value | ||||
| Age (<75/≥75 years) | 193/249 | 0.49 | 649/436 | 0.37 | |||||||||
| Sex (male/female) | 203/202 | 0.87 | 516/920 | 0.44 | |||||||||
| ECOG PS (0–1/2–3) | 246/68 | 0.001* | 1.334 | 0.6985–2.441 | 0.37 | 646/127 | 0.001 | 1.947 | 0.987–3.733 | 0.054 | |||
| Smoking (BI) (820</≥820) | 193/227 | 0.29 | 436/548 | 0.68 | |||||||||
| Histology (AC/non-AC) | 195/205 | 0.82 | 563/516 | 0.96 | |||||||||
| Lymph meta (yes/no) | 171/393 | 0.057 | 436/775 | 0.11 | |||||||||
| Brain meta (yes/no) | 255/195 | 0.28 | NR/515 | 0.14 | |||||||||
| Bone meta (yes/no) | 157/246 | 0.03* | 1.548 | 0.654–3.570 | 0.31 | 267/704 | 0.03 | 1.338 | 0.479–3.639 | 0.57 | |||
| NLR (high/low) (≥2.8/<2.8) | 143/331 | 0.003* | 1.703 | 1.041–2.906 | 0.03* | 367/NR | 0.001 | 2.246 | 1.157–4.727 | 0.01* | |||
| PD-L1 (%) (≥1/<1) | 196/202 | 0.22 | 571/416 | 0.14 | |||||||||
| SUVmax (high/low) | 208/193 | 0.81 | 516/548 | 0.68 | |||||||||
| SUVpeak (high/low) | 208/193 | 0.91 | 515/NR | 0.06 | |||||||||
| MTV (high/low) | 74/273 | 0.001* | 2.125 | 1.266–3.463 | 0.004* | 128/720 | 0.001 | 2.189 | 1.198–3.894 | 0.01* | |||
| TLG (high/low) | 151/720 | 0.001* | 151/720 | 0.001 | |||||||||
*, statistically significance. AC, adenocarcinoma; BI, brinkman index; CI, confidence interval; MST, median survival time (days); ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; lymph, lymph node; MTV, metabolic tumor volume; non-AC, non-adenocarcinoma; NR, not reached; NLR, neutrophil to lymphocyte ratio; NR, not reached; OS, overall survival; PD-L1, programmed death ligand-1; PFS, progression-free survival; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis.
Impact of outcomes according to different variables
Next, we examined the survival analysis based on histology (AC and non-AC) and PD-L1 expression (PD-L1 <1% versus PD-L1 ≥1%). The results of the sub-analyses for PFS and OS are presented as supplemental files (Tables S1-S4, online only). Among 70 patients with AC, the univariate analysis identified PS, MTV, and TLG as significant predictors for PFS, while PS, NLR, MTV, and TLG were significant predictors of OS (Table S1). In multivariate analysis, albumin, PS, and MTV were identified as independent prognostic factors of PFS and OS. The univariate analysis of 60 patients with non-AC revealed that NLR was significantly associated with worse PFS rates, while PS, NLR, MTV, and TLG were significant predictors of OS (Table S2). Only NLR was an independent prognostic factor for predicting worse PFS and OS in the multivariate analysis. Among the 51 patients with PD-L1 ≥1%, univariate analysis identified PS, MTV, and TLG as significant predictors of PFS, while PS, NLR, MTV, and TLG were significant predictors of OS (Table S3). Multivariate analysis confirmed that NLR was an independent predictor of PFS and OS. Among the 79 patients with PD-L1 <1%, the univariate analysis identified PS, bone metastases, MTV, and TLG as significant predictors of PFS and OS (Table S4). In contrast, multivariate analysis confirmed bone metastases and MTV as independent predictors of PFS and MTV for OS. The Kaplan-Meier survival curve for PFS based on MTV is shown in Figure 3.

Potential of predictors for baseline parameters based on MTV and PFS
We identified the baseline parameters according to MTV on 18F-FDG uptake in the groups with better and worse PFS (<6 and ≥6 months) (Table 3). Among patients with PFS <6 months, bone metastases, low albumin levels, and high CRP level were significantly associated with high MTV. In contrast, good PS, high albumin levels, and low CRP level were associated with low MTV in patients with PFS ≥6 months.
Table 3
| Different variables | PFS <6 months | PFS ≥6 months | |||||
|---|---|---|---|---|---|---|---|
| High MTV (n=27) | Low MTV (n=31) | P | High MTV (n=6) | Low MTV (n=66) | P | ||
| Age (<75/≥75 years) | 19/8 | 24/7 | 0.56 | 6/0 | 37/29 | 0.07 | |
| Sex (male/female) | 24/3 | 23/8 | 0.19 | 6/0 | 54/12 | 0.58 | |
| ECOG PS (0–1/2–3) | 17/10 | 26/5 | 0.08 | 4/2 | 65/1 | 0.01* | |
| Smoking (BI) (820</≥820) | 12/15 | 15/16 | 0.79 | 4/2 | 39/27 | >0.99 | |
| Histology (AC/non-AC) | 17/10 | 16/15 | 0.43 | 1/5 | 36/30 | 0.10 | |
| Lymph meta (yes/no) | 25/2 | 26/5 | 0.43 | 2/4 | 46/20 | 0.09 | |
| Brain meta (yes/no) | 5/22 | 3/28 | 0.45 | 1/5 | 13/53 | >0.99 | |
| Bone meta (yes/no) | 19/8 | 6/25 | 0.001* | 2/4 | 19/47 | >0.99 | |
| PD-L1 (%) (≥1/<1) | 10/17 | 12/19 | >0.99 | 3/3 | 25/41 | 0.67 | |
| NLR (high/low) (≥2.8/<2.8) | 25/2 | 27/4 | 0.67 | 5/1 | 41/25 | 0.40 | |
| Leukocyte (high/low) | 17/10 | 13/18 | 0.12 | 5/1 | 31/35 | 0.19 | |
| Neutrophil (high/low) | 18/9 | 16/15 | 0.29 | 5/1 | 27/39 | 0.08 | |
| Lymphocyte (high/low) | 10/17 | 11/20 | >0.99 | 5/1 | 39/27 | 0.39 | |
| Albumin (high/low) | 3/24 | 16/15 | 0.001* | 1/5 | 44/22 | 0.02* | |
| CRP (high/low) | 22/5 | 17/14 | 0.049* | 5/1 | 21/45 | 0.02* | |
| RT before Nivo-Ipi (yes/no) | 10/17 | 12/19 | >0.99 | 1/5 | 15/51 | >0.99 | |
*, statistically significance. BI, Brinkman index; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; lymph, lymph node; meta, metastasis; MTV, metabolic tumor volume; NLR, neutrophil to lymphocyte ratio; PD-L1, programmed death ligand-1; Prior RT, radiation before initial treatment; PFS, progression-free survival; PS, performance status.
Comparison of patient demographics between patients with worse outcomes (high MTV/PFS <6 months) and favorable prognosis (low MTV/PFS ≥12 months) revealed that poor PS, lymph node metastases, bone metastasis, high NLR, high neutrophil counts, low lymphocyte counts, low albumin levels, and high CRP level were significantly related to the worse outcome (Table S5, online only).
Discussion
The results of the current study identified MTV based on 18F-FDG uptake as a significant predictor for Nivo-Ipi in patients with advanced NSCLC. Although four indicators—SUVmax, SUVpeak, MTV, and TLG—can be used to assess 18F-FDG uptake within tumor tissues, metabolic tumor activity determined by MTV was the most promising biomarker for predicting outcomes after Nivo-Ipi treatment. High MTV at baseline was closely related to factors negatively associated with ICI efficacy such as high NLR, high CRP level, low albumin levels, poor PS, non-PR, and decreasing irAE incidence. Additionally, the results of sub-analyses according to histology and PD-L1 expression identified MTV as an independent prognostic predictor for Nivo-Ipi treatment in patients with histological findings of AC and PD-L1 <1%. Despite a high MTV, the presence of lymph node metastases was closely related to shorter PFS; however, poor PS, high NLR, low lymphocyte counts, and high CRP level were negative predictors for worse PFS in patients with low MTV. Our results indicated that high MTV with PFS <6 months was associated with poor PS, high NLR, low albumin levels, and high CRP level. However, high MTV was a superior predictor than poor PS and high NLR for predicting Nivo-Ipi outcome in patients with PD-L1 <1%. The follow-up period of our eligible samples was inadequate for the assessment of 5-year survival after Nivo-Ipi therapy; thus, further investigation is warranted to elucidate the potential prediction of long-term survival according to pretreatment MTV.
We recently reported that pretreatment tumor metabolic activity by MTV and TLG, but not maximal level by SUVmax, was a significant predictor of anti-PD-1 antibody monotherapy or combined chemotherapy with PD-1 blockade (3-5). In their systematic review and meta-analysis, Ling et al. reported the prognostic value of 18F-FDG PET in patients with metastatic or advanced NSCLC who received PD-1 blockade except for anti-CTLA4 antibody (12). This meta-analysis included 13 eligible studies (743 patients) among 540 relevant articles. The results confirmed that baseline MTV and TLG, but not SUVmax and SUVmean, may have predictive value (12). MTV and TLG accurately reflect tumor aggressiveness by measuring tumor volume and metabolic activity in three dimensions, and high 18F-FDG uptake may be associated with hypoxia or tumor necrosis. Tumor necrosis and hypoxia could regulate the immunosuppressive tumor microenvironment by promoting immunosuppressive cells, including myeloid-derived suppressor cells, regulatory T cells (Tregs), and tumor-associated macrophages (13,14). Previous studies reported that forkhead box P3 (FOXP3), a major regulator of Tregs, is closely correlated with MTV (15,16). The correlation between PD-L1 expression and MTV differs among studies; thus, the association of MTV with PD-L1 expression remains unclear (17,18). Previous evidence suggests that environments with high MTV form an immunosuppressive context, leading to ICI resistance, regardless of PD-L1 expression. The results of the present study demonstrated that MTV was closely correlated with patient nutrition, inflammation, and general condition, leading to worse outcomes and therapeutic resistance. While MTV is a significant biomarker for predicting ICI response and prognosis, the mechanisms of these effects remain unknown (12).
The addition of anti-CTLA4 antibody to PD-1 blockade provides consistent efficacy for patients with NSCLC regardless of PD-L1 expression level (1,2), while the therapeutic efficacy of PD-1 blockade monotherapy is affected by PD-L1 expression level (18). We believe that the prognostic role of predictive parameters differs between Nivo-Ipi and PD-1 blockade monotherapy. In patients with PD-L1<1%, dynamically assessing the status of the tumor immune environment is challenging; therefore, metabolic tumor activity based on 18F-FDG uptake may be a promising predictor to dynamically assess the tumor microenvironment in Nivo-Ipi treatment. Although the reasons why metabolic tumor activity is predictive of Nivo-Ipi therapy on the tumor microenvironment with PD-L1 <1% remain unclear, our results suggest that MTV on 18F-FDG uptake may be useful as a more powerful predictor in patients with PD-L1 <1%.
The present study has several limitations. First, while our sample size was relatively sufficient compared with previous studies, the numbers of patients in the sub-analyses according to different parameters were limited, which may have biased our results. In particular, the prognostic potential of MTV requires additional investigation in a larger population of patients with PD-L1 <1%. Although MTV reflects the glycometabolism of whole tumor lesions, the metabolic tumor activity according to location of metastases or number of involved organs was unable to be assessed by MTV on PET. However, the assessment of SUVmax in different sites according to location of metastases or number of involved organs is available. As SUVmax could not predict the outcome after immunotherapy, it may bias to measure the value of SUVmax in different tumor lesions for prognostic prediction. Although this approach may be difficult to improve the results of our data, it is crucial to analyze the predictive potential of 18F-FDG accumulation based on individual malignant lesions as next study. Second, the trend of tumor-infiltrating lymphocytes (TILs) plays a crucial role in the effectiveness of Nivo-Ipi treatment. The present study did not examine the relationship between 18F-FDG uptake and TILs for the prognostic prediction of Nivo-Ipi. Therefore, future studies are needed to evaluate the predictive potential of 18F-FDG uptake correlated with TILs. Finally, while the results of the present study demonstrated that MTV was predictive of the outcome after Nivo-Ipi therapy, whether MTV can predict the therapeutic efficacy of anti-CTLA4 antibody alone remains unclear. Further investigation is warranted to evaluate the association between 18F-FDG uptake and CTLA4 function.
Conclusions
MTV determined according to 18F-FDG uptake was identified as a negative prognostic factor after Nivo-Ipi treatment in patients with advanced NSCLC, especially in those with PD-L1 <1%. MTV based on 18F-FDG uptake may be useful for predicting long-term survival after Nivo-Ipi treatment.
Acknowledgments
The authors thank Ms. Kozue Watanabe, Ms. Saki Toita, and Ms. Koko Kodaira for their assistance in preparing the manuscript. The authors also thank Editage (www.editage.jp) for English language editing.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1084/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1084/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1084/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1084/coif). K.K. received the funding from JSPS Grant-in-Aid for Scientific Research C (No. 24K10292) and a speaker honorarium from Ono Pharmaceutical Company, Chugai Pharmaceutical, and AstraZeneca and research grants from AstraZeneca. A.M. and O.Y. received speaker honoraria from Chugai Pharmaceutical and AstraZeneca, respectively. H.K. received research grants from Ono Pharmaceutical Company, Bristol-Myers Company, Chugai Pharmaceutical and speaker honoraria from Ono Pharmaceutical Company, Bristol-Myers Company, MSD, Chugai Pharmaceutical, and AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Ethics Committee of the International Medical Center of Saitama Medical University (No. 20-125), which waived the requirement for written informed consent owing to the retrospective study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brahmer JR, Lee JS, Ciuleanu TE, et al. Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J Clin Oncol 2023;41:1200-12. [Crossref] [PubMed]
- Reck M, Ciuleanu TE, Schenker M, et al. Five-year outcomes with first-line (1L) nivolumab+ipilimumab+chemotherapy (N+I+C) vs C in patients (pts) with metastatic NSCLC (mNSCLC) in CheckMate 9LA. J Clin Oncol 2024;42:8560.
- Yamaguchi O, Kaira K, Hashimoto K, et al. Tumor metabolic volume by 18F-FDG-PET as a prognostic predictor of first-line pembrolizumab for NSCLC patients with PD-L1 ≥ 50. Sci Rep 2020;10:14990. [Crossref] [PubMed]
- Hashimoto K, Kaira K, Yamaguchi O, et al. Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer. J Clin Med 2020;9:725. [Crossref] [PubMed]
- Hashimoto K, Kaira K, Imai H, et al. Prognostic Potential of Metabolic Activity on 18 F-FDG Accumulation in Advanced NSCLC Receiving Combining Chemotherapy Plus PD-1 Blockade. J Immunother 2022;45:349-57. [Crossref] [PubMed]
- Iravani A, Wallace R, Lo SN, et al. FDG PET/CT Prognostic Markers in Patients with Advanced Melanoma Treated with Ipilimumab and Nivolumab. Radiology 2023;307:e221180. [Crossref] [PubMed]
- Iravani A, Osman MM, Weppler AM, et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur J Nucl Med Mol Imaging 2020;47:2776-86. [Crossref] [PubMed]
- Kaira K, Kuji I, Kagamu H. Value of (18)F-FDG-PET to predict PD-L1 expression and outcomes of PD-1 inhibition therapy in human cancers. Cancer Imaging 2021;21:11. [Crossref] [PubMed]
- Yamaguchi O, Kaira K, Imai H, et al. Clinical Utility of Inflammatory and Nutritious Index as Therapeutic Prediction of Nivolumab plus Ipilimumab in Advanced Non-Small Cell Lung Cancer. Oncology 2024;102:271-82. [Crossref] [PubMed]
- Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol 2022;52:539-44. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Ling T, Zhang L, Peng R, et al. Prognostic value of 18F-FDG PET/CT in patients with advanced or metastatic non-small-cell lung cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front Immunol 2022;13:1014063. [Crossref] [PubMed]
- Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 2014;143:512-9. [Crossref] [PubMed]
- Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011;475:226-30. [Crossref] [PubMed]
- Schaaf MB, Garg AD, Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis 2018;9:115. [Crossref] [PubMed]
- Wang Y, Zhao N, Wu Z, et al. New insight on the correlation of metabolic status on (18)F-FDG PET/CT with immune marker expression in patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2020;47:1127-36. [Crossref] [PubMed]
- Jreige M, Letovanec I, Chaba K, et al. (18)F-FDG PET metabolic-to-morphological volume ratio predicts PD-L1 tumour expression and response to PD-1 blockade in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2019;46:1859-68. [Crossref] [PubMed]
- Wang D, Li Y, Chen X, Li P. Prognostic significance of volume-based 18F-FDG PET/CT parameters and correlation with PD-L1 expression in patients with surgically resected lung adenocarcinoma. Medicine (Baltimore) 2021;100:e27100. [Crossref] [PubMed]


